In Vivo DMPK Service
Drug metabolism and pharmacokinetics (DMPK) form a cornerstone of preclinical drug development. In vivo DMPK studies are essential for understanding the absorption, distribution, metabolism, and excretion (ADME) of drug candidates in living organisms. Unlike in vitro assays, which provide preliminary screening data, in vivo studies offer direct insights into systemic exposure, bioavailability, half-life, clearance, and tissue distribution under physiological conditions. These studies help predict human pharmacokinetics, evaluate dose proportionality, assess potential drug-drug interactions, and guide formulation optimization. In vivo DMPK data provide critical support across all stages of drug development.
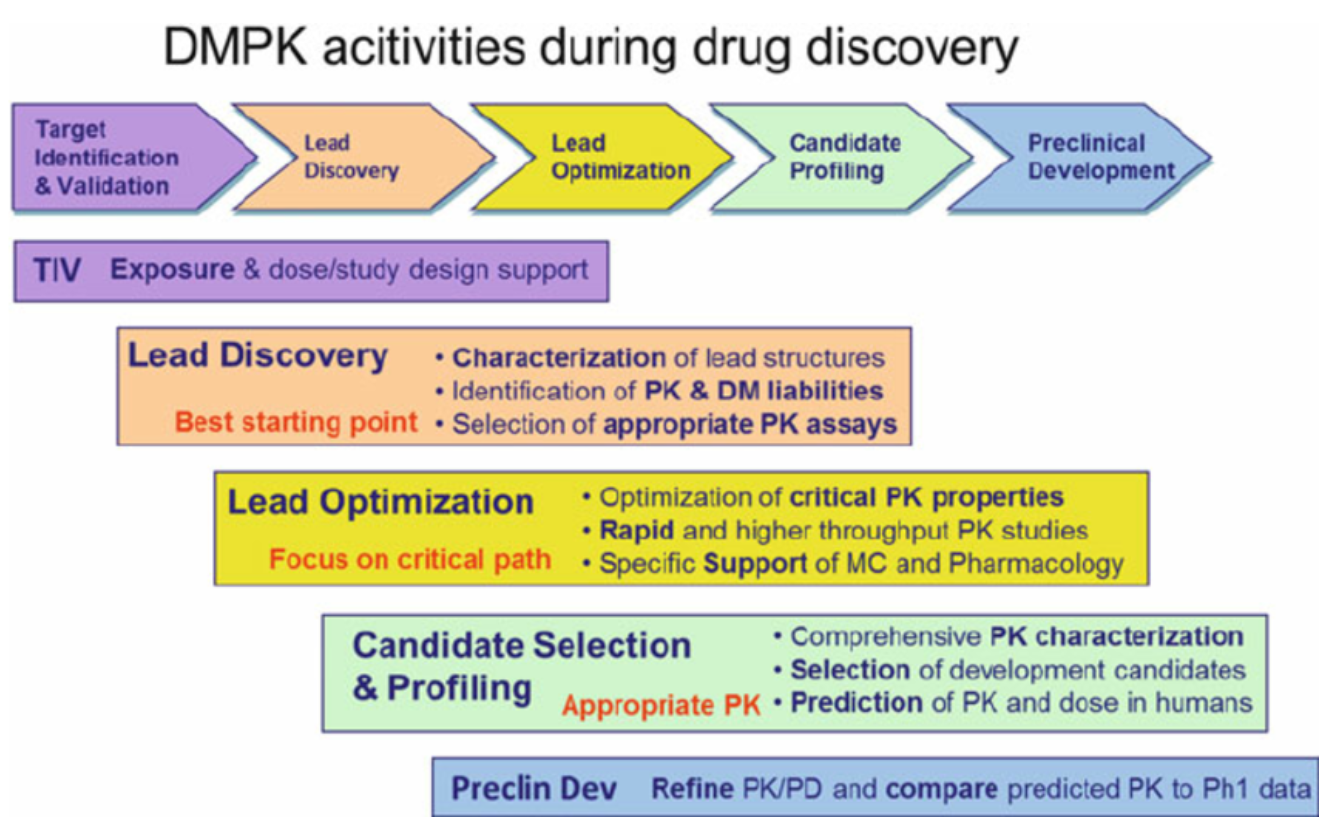
Figure 1. Overview of the Main Tasks of DMPK Support during the Different Phases of Drug Discovery
(TIV: Target Identification and Validation, PK: Pharmacokinetics, DM: Drug Metabolism, MC: Medicinal Chemistry)
Service at MtoZ Biolabs
MtoZ Biolabs provides end-to-end In Vivo DMPK Services tailored to pharmaceutical, biotechnology, and academic partners worldwide. Leveraging our state-of-the-art LC-MS/MS platforms, internationally compliant animal facilities, and cross-disciplinary expertise, we deliver high-quality, reproducible, and regulation-ready data to support your decision-making process. We support a wide range of animal species (mice, rats, dogs) and administration routes (IV, PO, SC, IP, IM), offering flexible and customizable study designs based on your project goals and compound characteristics.
Our In Vivo DMPK Services Include:
💠PK Screening: Rapid evaluation of systemic exposure, half-life, and clearance across multiple candidates.
💠Tissue Distribution Studies: Quantify drug levels in plasma, brain, liver, kidney, and other organs.
💠BBB Penetration Studies: Assess compound exposure in cerebrospinal fluid and across the blood-brain barrier.
💠Excretion Analysis: Metabolic cages are used to collect and quantify urinary and fecal excretion of test compounds.
💠Mass Balance Studies: Track radiolabeled compounds to quantify total recovery and distribution across absorption, metabolism, and excretion pathways.
💠Metabolite Profiling: Comprehensive in vivo analysis of major circulating and excreted metabolites to support metabolic pathway elucidation.
In addition to in vivo DMPK studies, MtoZ Biolabs also offers a comprehensive range of In Vitro ADME Services, comprising assays such as permeability, solubility, metabolic stability, and drug–drug interaction testing, enabling clients to build a seamless preclinical pharmacokinetic evaluation pipeline.
Analysis Workflow
1. Study Design and Dosing Strategy
We develop customized study protocols based on compound properties and research objectives, selecting appropriate dosing routes (e.g., IV, oral) and schedules.
2. Sample Collection
Biological samples such as plasma, tissues, cerebrospinal fluid (CSF), urine, and feces are collected at predefined intervals to capture the full pharmacokinetic profile.
3. Quantitative Bioanalysis
Samples are analyzed using LC-MS/MS or UPLC-HR/MS. Analytical methods are developed and validated specifically for each compound to ensure sensitivity and specificity.
4. Pharmacokinetic Evaluation
Concentration-time data are processed using non-compartmental or compartmental modeling to extract key PK parameters.
5. Metabolite Profiling and Mass Balance
When needed, LC-HR/MS data are leveraged to identify in vivo metabolites. Radiolabeled studies can be included to assess total recovery and compound fate.
6. Data Reporting
Final deliverables include full PK reports, raw data, and interpretation to support lead optimization or regulatory submission.
Service Advantages
✅ High-sensitivity LC-MS/MS platforms for accurate bioanalysis
✅ Experienced multidisciplinary team with deep DMPK expertise
✅ Flexible study design tailored to your project goals
✅ One-stop service from study design to data analysis, saving your time and effort.
Deliverables
1. Study protocol and analytical setup
2. Raw Data Files
3. Processed results and DMPK parameters
4. Data visualization with interpretation summaries
5. Summary report outlining key findings
Partner with MtoZ Biolabs for reliable and insightful in vivo DMPK studies. Contact us today to discuss your project needs and receive a customized study plan!
Related Services
Lead Optimization Service | Drug Discovery
Hit-to-Lead Identification Service | Drug Discovery
Hit Identification Service | Drug Discovery
Target Identification and Validation Service| Drug Discovery
How to order?







