In Vitro ADME Service
- Kinetic Solubility: Measures the apparent solubility of a compound under non-equilibrium conditions, providing a rapid screen for formulation feasibility.
- Thermodynamic Solubility: Determines the true solubility at equilibrium, essential for calculating bioavailability and guiding salt selection strategies.
- LogD Measurement: Quantifies the distribution coefficient at physiological pH, predicting membrane permeability and tissue penetration.
- EPSA (Exposed Polar Surface Area): Assesses polar surface coverage, which correlates with oral absorption, blood–brain barrier penetration, and P-gp susceptibility.
- Microsomal Stability: Assesses metabolic stability using liver microsomes to estimate hepatic clearance and potential for oxidative metabolism.
- Hepatocyte Stability: Measures metabolic processes using hepatocytes, evaluating clearance rates.
- Plasma Stability: Determines the compound's susceptibility to hydrolysis or enzymatic degradation in plasma.
- Blood Stability: Evaluates compound integrity in whole blood to identify potential degradation or binding events that may affect pharmacokinetic interpretation.
- Chemical Stability: Tests compound behavior in buffer systems under varying pH conditions to assess chemical degradation risks independent of enzymatic pathways.
ADME, which stands for Absorption, Distribution, Metabolism, and Excretion, describes the pharmacokinetic behavior of a drug within a biological system. Understanding a compound’s ADME profile is fundamental to determining its safety, efficacy, and dosing regimen. These properties collectively influence a drug’s bioavailability, half-life, and potential for drug–drug interactions.
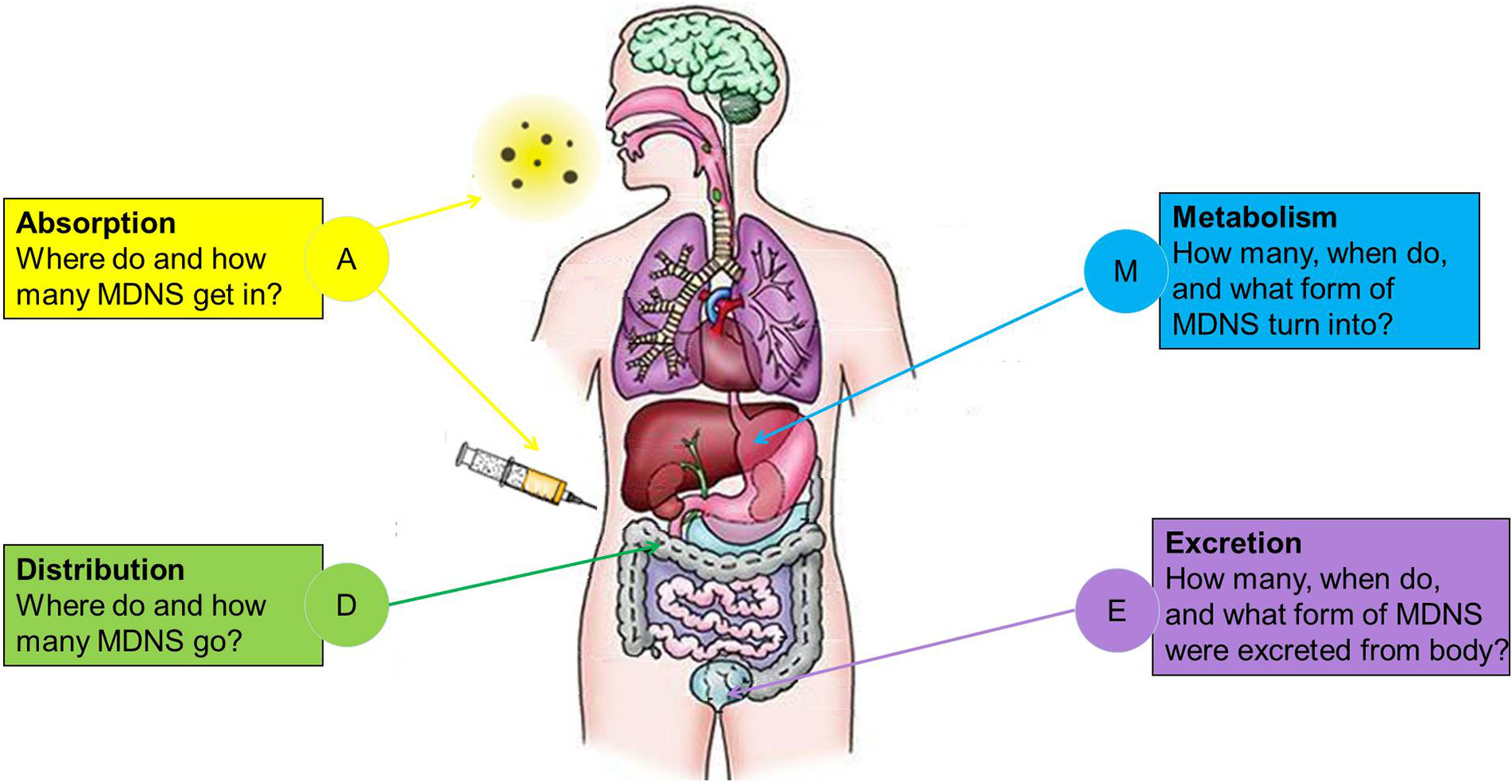
Figure 1. ADME Processes of MDNS in Vivo. (MDNS: Materials of the Drug Nanocarrier System)
In vitro ADME studies are laboratory-based assays that simulate human physiological conditions to predict how a compound will behave in vivo—before advancing to animal models or clinical trials. Compared with in vivo studies, in vitro ADME testing offers a faster, cost-effective, and ethical approach to screening multiple drug candidates during early-phase development. In vitro ADME testing helps reduce attrition rates by eliminating candidates with poor bioavailability, metabolic instability, or toxicity risks. MtoZ Biolabs offers a comprehensive In Vitro ADME Service designed to generate reliable early-stage pharmacokinetic data and support informed decision-making in drug discovery.
Service at MtoZ Biolabs
MtoZ Biolabs' In Vitro ADME Service integrates industry-standard assays with cutting-edge analytical platforms to support rational drug design, screening, and safety evaluation.
1. Physicochemical Properties Characterization
Understanding a compound’s fundamental physical and chemical traits is critical for predicting its absorption and formulation behavior.
💠Solubility Testing: Quantifies compound solubility in aqueous buffers at different pH values to predict bioavailability and formulation challenges.
💠Lipophilicity Profiling: Determines compound’s affinity for lipid environments, indicating membrane permeability and tissue accumulation potential.
2. Absorption Assessment
To evaluate how a compound crosses biological membranes, MtoZ Biolabs offers a range of absorption models:
💠Skin Permeation Studies: Assess dermal absorption using human or animal skin membranes, particularly useful for transdermal delivery systems.
💠PAMPA (Parallel Artificial Membrane Permeability Assay): Simulates passive transcellular diffusion through lipid bilayers, offering a cost-effective permeability screen.
💠Caco-2 Permeability Assay: Employs human intestinal epithelial cells to evaluate both passive diffusion and active transport, considered the gold standard for intestinal absorption prediction.
💠MDCK Permeability Assay: Uses MDR1- or BCRP-transfected kidney cells to assess epithelial and blood-brain barrier (BBB) permeability relevant to brain and GI drug distribution.
3. Drug Distribution Profiling
We evaluate how drug candidates partition within biological matrices, providing insights into plasma retention, tissue penetration, and systemic availability.
💠Plasma Protein Binding: Measures the free and bound drug fractions using equilibrium dialysis or ultrafiltration. Free drug concentration correlates with pharmacological activity.
💠Tissue Binding Analysis: Assesses compound affinity to tissue homogenates, predicting distribution volume and target tissue exposure.
💠Plasma Partitioning: Evaluates compound distribution between plasma and blood cells, affecting systemic clearance rates.
💠Microparticle Binding Assessment: Measures drug interaction with particulate carriers or endogenous vesicles, especially relevant in nanoparticle formulations.
4. In Vitro Metabolism Analysis
Metabolism studies help predict biotransformation pathways, metabolic liabilities, and clearance potential.
💠Metabolite Identification and Profiling: Employs high-resolution LC-MS/MS to characterize primary and secondary metabolites across human and preclinical systems.
💠Metabolic Stability Assays: Metabolic stability plays a critical role in determining the in vivo half-life, clearance rate, and systemic exposure of drug candidates. Poor metabolic stability often leads to insufficient bioavailability and rapid elimination, resulting in suboptimal therapeutic outcomes or increased dosing frequency.
5. Drug–Drug Interaction (DDI) Risk Evaluation
MtoZ Biolabs performs a range of in vitro assays to identify DDI liabilities, supporting regulatory compliance and clinical safety.
💠CYP Inhibition Studies: Determine whether a compound inhibits major cytochrome P450 isoforms, either reversibly or via time-dependent inhibition (TDI), potentially leading to elevated exposure of co-administered drugs.
💠CYP Induction Assays: Assess upregulation of CYP gene expression, typically via PXR or CAR activation, using primary hepatocyte cultures.
💠CYP Reaction Phenotyping Assays: Identifies the specific CYP enzymes responsible for compound metabolism, allowing for better prediction of inter-individual metabolic variability and DDI susceptibility.
Why Choose MtoZ Biolabs?
✅State-of-the-art In Vitro ADME Service platform
✅Highly experienced team in pharmacokinetics and drug metabolism
✅Full-spectrum ADME services under one roof
✅Fast turnaround with high reproducibility
✅Customizable workflows tailored to your molecule type and study phase
What Could be Included in the Report?
1. Assay protocols and experimental conditions
2. Raw and processed data files
3. Parameter interpretation (e.g., permeability coefficients, metabolic half-lives, binding rates)
4. Full report with key takeaways
5. Recommendations for lead optimization or further testing
MtoZ Biolabs is committed to accelerating drug discovery through precise and reliable ADME insights. Our In Vitro ADME Service empowers your team with timely, high-quality pharmacokinetic data—helping you select, refine, and advance the best candidates for clinical development. Contact us today to discuss your ADME profiling needs.
Related Services
Lead Optimization Service | Drug Discovery
Hit-to-Lead Identification Service | Drug Discovery
Hit Identification Service | Drug Discovery
Target Identification and Validation Service| Drug Discovery
How to order?







