Glycoprotein Separation and Purification Service
- Professional expertise in glycoprotein chemistry and biopharmaceutical analysis
- Precise separation and purification ensuring high sample quality and reproducibility
- Efficient workflows optimized for speed without compromising data accuracy
- Flexible approaches tailored to different sample types and research objectives
- Integration with downstream structural, functional, and sequencing analyses
- High sensitivity enabling detection and purification of low-abundance glycoproteins
Glycoproteins form a structurally diverse group of biomolecules that are distinguished by the covalent attachment of glycans to specific amino acid residues within the protein backbone. These carbohydrate chains vary in length, branching, and monosaccharide composition, resulting in a wide spectrum of structural features that directly influence glycoprotein function. A hallmark of glycoproteins is their high degree of heterogeneity. Differences can arise from the type of sugars incorporated, the linkages between them, and the number and position of glycosylation sites. This structural diversity significantly contributes to their biological roles but also makes their isolation and characterization particularly complex.
The challenges are further compounded by the fact that glycoproteins are often present in low concentrations within biological samples and are accompanied by abundant interfering molecules such as lipids, nucleic acids, and non-glycosylated proteins. Additionally, glycoproteins are structurally delicate and highly responsive to changes in their surrounding environment. Variations in temperature, pH, or ionic strength can easily disrupt their three-dimensional conformation, leading to loss of activity or aggregation.
Because of this intrinsic complexity and sensitivity, glycoprotein separation and purification are indispensable steps in analytical workflows. Without effective isolation, subtle glycosylation differences may be overlooked, and conventional analytical methods may fail to distinguish among closely related isoforms. Therefore, specialized purification strategies combined with advanced analytical technologies are essential for achieving reliable structural and functional insights into glycoproteins.
Service at MtoZ Biolabs
MtoZ Biolabs offers a dedicated Glycoprotein Separation and Purification Service designed to address the complexity and heterogeneity of glycoproteins. By integrating advanced separation strategies, purification workflows, and state-of-the-art analytical technologies, we provide reliable and reproducible data that support both basic research and biopharmaceutical development. Our service ensures that clients obtain high-quality glycoprotein samples with preserved structural integrity for downstream characterization and functional studies.
1. Separation Techniques
🔸Size-Exclusion Chromatography (SEC): Separates glycoproteins based on molecular size, allowing resolution of intact proteins and removal of aggregates or contaminants.
🔸Ion-Exchange Chromatography (IEX): Exploits charge differences to separate glycoproteins and glycoforms, offering fine resolution of isoforms with distinct glycosylation.
🔸Affinity Chromatography: Uses specific ligands such as lectins or antibodies to selectively capture glycoproteins containing defined glycan structures or epitopes.
🔸Gel Electrophoresis: Provides molecular weight-based separation and visualization of glycoproteins, supporting qualitative assessment and preparative isolation.
2. Purification Techniques
🔸Affinity Purification: Achieves high selectivity by isolating target glycoproteins through ligand binding, often used for therapeutic protein preparation.
🔸Hydrophobic Interaction Chromatography (HIC): Separates glycoproteins based on hydrophobicity, complementing other chromatographic methods.
🔸Reversed-Phase Chromatography (RPC): Useful for peptide-level separation and glycopeptide purification prior to mass spectrometry analysis.
🔸Precipitation Methods: Enables bulk purification or enrichment of glycoproteins under controlled solvent or salt conditions.
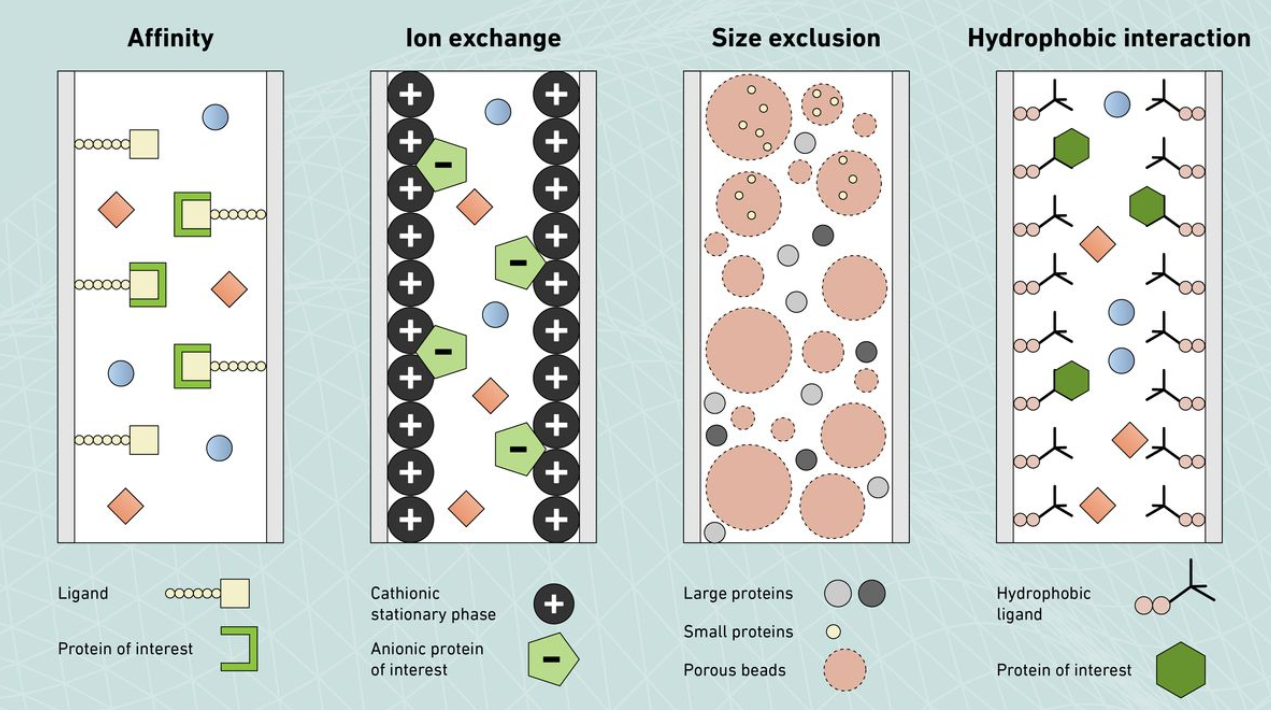
Credit: Technology Networks.
Figure 1. Scheme of the Columns Employed in Some of the Typical Chromatography Techniques Used for Protein Separation and Purification
3. Downstream Analysis after Separation and Purification
At MtoZ Biolabs, glycoprotein separation and purification are not the end of the workflow but the foundation for further exploration. Once samples are isolated with high purity, we provide a wide range of downstream analytical options to extract meaningful biological and functional information. These include:
🔸Sequencing and Mapping: Amino acid sequencing combined with site-specific glycosylation mapping to provide a complete molecular picture of the glycoprotein. This includes verification of protein sequence integrity and detection of modifications.
🔸Quantitative Analysis: Determination of glycoprotein abundance, relative glycoform distribution, and batch-to-batch consistency for both research and industrial applications.
🔸Structural Characterization: Detailed assessment of glycan structures, glycosylation sites, and protein backbones to understand molecular heterogeneity.
🔸Functional Evaluation: Linking structural information with biological function, such as stability, receptor binding, or immunogenic potential.
Service Advantages
Applications
1. Medical Research
Glycoprotein separation and purification allow scientists to isolate low-abundance glycoproteins and study their structural and functional roles in health and disease. This service enables the discovery of disease-associated glycoprotein variants, supports biomarker validation, and helps clarify mechanisms of conditions such as cancer, autoimmune disorders, and neurodegenerative diseases.
2. Drug Development
In the pharmaceutical field, therapeutic proteins including monoclonal antibodies, Fc-fusion proteins, and glycoprotein-based vaccines require precise glycosylation control. Our service provides high-quality purified glycoproteins for in-depth analysis, ensuring batch-to-batch consistency, improved drug stability, and regulatory compliance. By resolving glycoform heterogeneity, it accelerates candidate selection, formulation optimization, and quality assurance in the drug development pipeline.
3. Food Industry
Glycoproteins are important components of many food products, influencing texture, nutritional value, and allergenicity. Our separation and purification workflows help characterize food-derived glycoproteins, enabling studies on food quality, safety testing, and allergen detection. This ensures compliance with food regulations and supports the development of functional foods with enhanced health benefits.
4. Agriculture
In agricultural research, glycoproteins play critical roles in plant defense mechanisms, seed development, and stress responses. Glycoprotein Separation and Purification Services provide researchers with the tools to study these biomolecules in detail, facilitating the identification of glycoproteins linked to crop resistance, yield improvement, and environmental adaptation. This contributes to advances in sustainable agriculture and crop biotechnology.
Sample Submission Suggestions
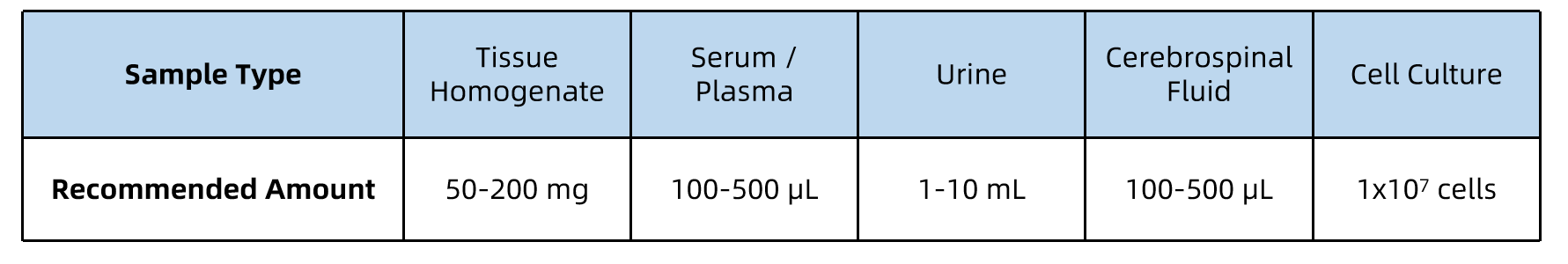
All samples should be kept on ice during processing, and stored at –80°C prior to shipment. Ship samples on dry ice with proper labeling and accompanying documentation.
If you have special sample types or require additional guidance, please contact us for personalized support before sample preparation.
Deliverables
1. Detailed description of experimental design and workflows.
2. Information on purification methods and separation conditions.
3. Chromatograms, electropherograms, and MS data for purified fractions.
4. Structural characterization of glycoproteins and glycopeptides.
5. Glycan profiles, site-specific glycosylation data, and quantitative analysis.
6. Raw and processed data files.
7. Comprehensive interpretation report.
Related Services
Glycoprotein Profiling Service
Intact Glycoproteins Analysis Service
Glycoprotein Amino Acid Sequence Analysis Service
Glycoprotein Quantification Service
How to order?







