Glycoprotein LC-MS Disulfide Bridge Mapping Service
Disulfide bridges are covalent bonds formed between the sulfur atoms of cysteine residues, serving as essential stabilizing elements in protein structures. In glycoproteins, these bonds help maintain correct folding, protect against denaturation, and preserve the three-dimensional conformation required for biological function. Proper disulfide connectivity ensures that glycosylation sites are positioned and oriented correctly, influencing interactions with other biomolecules and affecting the overall functionality of the glycoprotein. Disruption or mispairing of disulfide bonds can lead to altered structural integrity, reduced stability, and compromised biological performance. Glycoprotein disulfide bridge mapping is the systematic analytical process of identifying and confirming the connectivity between cysteine residues in a glycoprotein. It involves determining the exact pairing of cysteines, detecting the presence of alternative or scrambled bonds, and identifying free cysteines. Such mapping provides crucial information about the structural integrity and folding of the glycoprotein, helping ensure that it meets quality and performance requirements.
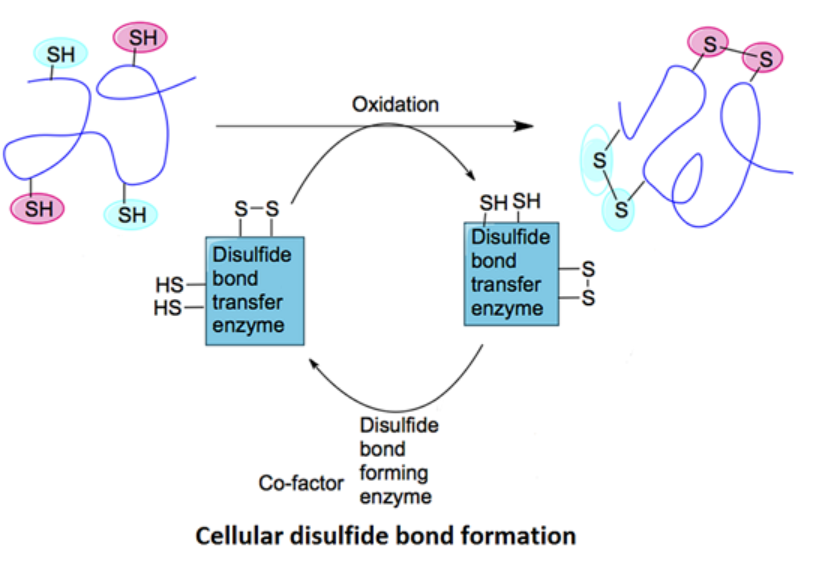
Figure 1. Cellular Disulfide Bridge Formation in Bioactive Proteins
Disulfide bridge mapping can be technically challenging due to the complexity of glycoprotein structures, the presence of multiple potential cysteine pairings, and the susceptibility of disulfide bonds to rearrangement during sample preparation. Traditional structural biology techniques such as X-ray crystallography and nuclear magnetic resonance (NMR) spectroscopy, while powerful, have limitations in resolving detailed disulfide connectivity, particularly in cases involving conformational flexibility, heterogeneous glycosylation, or low sample availability. These challenges necessitate the use of specialized analytical approaches, with liquid chromatography–mass spectrometry (LC-MS) being one of the most powerful and versatile methods available.
Service at MtoZ Biolabs
MtoZ Biolabs offers a dedicated Glycoprotein LC-MS Disulfide Bridge Mapping Service designed to accurately characterize disulfide connectivity in glycoproteins. Our platform combines optimized sample preparation workflows with advanced chromatographic separation and high-resolution tandem mass spectrometry (LC-MS/MS) to preserve and precisely map native disulfide bonds, even in complex or heterogeneous glycoproteins. We also integrate isotope labeling quantitation methods such as Tandem Mass Tag (TMT) and Isobaric Tags for Relative and Absolute Quantitation (iTRAQ) to provide robust quantitative comparisons, and employ specialized bioinformatics pipelines for confident data interpretation.
Our service can provide:
🔸Identification of Native Disulfide Linkages: Confirm expected cysteine–cysteine pairings and validate that disulfide bridges are correctly formed.
🔸Detection of Disulfide Heterogeneity: Identify alternative disulfide patterns, scrambled bonds, or free cysteines that may indicate folding irregularities.
🔸Quantitative Assessment of Disulfide Occupancy: Measure the proportion of correctly formed versus altered or missing disulfide bonds.
🔸Correlation with Glycosylation Features: Integrate disulfide mapping data with glycosylation analysis to evaluate structural–functional relationships.
🔸Comparative Studies: Compare disulfide connectivity across different production batches, manufacturing processes, or glycoprotein variants.
Analysis Workflow
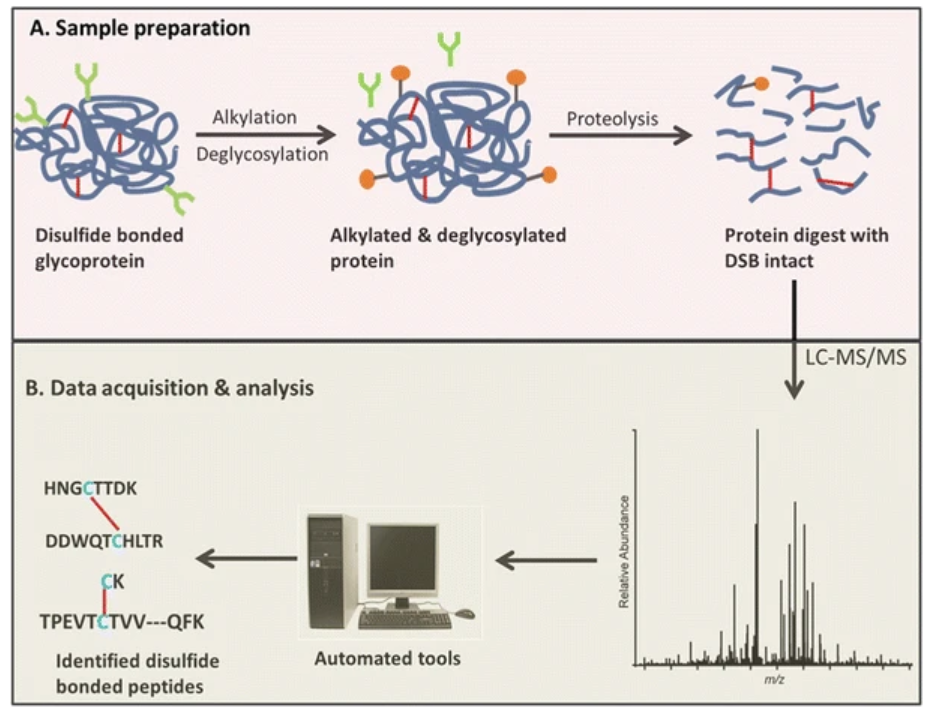
Figure 2. Workflow for Glycoprotein LC-MS Disulfide Bridge Mapping Service
Service Advantages
✔️Professional: Delivered by a team of experienced scientists with extensive expertise in glycoprotein structural analysis and LC-MS workflows.
✔️Precise: Advanced chromatographic separation, high-resolution tandem mass spectrometry, and robust bioinformatics ensure accurate disulfide mapping results.
✔️Efficient: Optimized protocols and data processing deliver high-quality results within competitive turnaround times.
✔️Flexible: Customizable workflows tailored to different glycoprotein types, sample conditions, and specific project objectives.
Applications
1. Biopharmaceutical Development
Verification of correct disulfide connectivity to ensure structural integrity, stability, and biological activity of therapeutic glycoproteins.
2. Quality Control and Batch Release
Routine monitoring of disulfide bond patterns to maintain batch-to-batch consistency in manufacturing.
3. Biosimilarity and Comparability Studies
Comparative mapping of disulfide bridges between biosimilars and reference products for regulatory submissions.
4. Protein Engineering and Design
Evaluation of engineered disulfide bonds to enhance stability, folding efficiency, or functional performance.
5. Structural and Mechanistic Studies
Investigation of disulfide bond roles in protein folding pathways, conformational stability, and functional regulation.
Sample Submission Suggestions
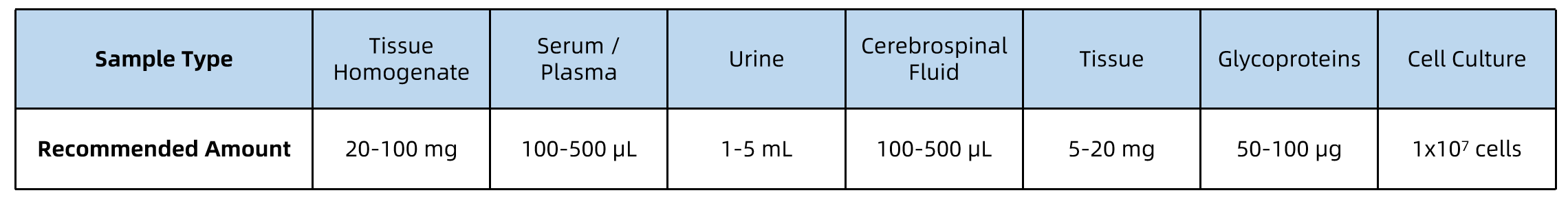
All samples should be collected under sterile conditions, kept on ice during processing, and stored at –80°C prior to shipment. Ship samples on dry ice with proper labeling and accompanying documentation.
If you have special sample types or require additional guidance, please contact us for personalized support before sample preparation.
Deliverables
1. Comprehensive Experimental Details
2. Materials, Instruments, and Methods
3. Total Ion Chromatogram & Quality Control Assessment (project-dependent)
4. Data Analysis, Preprocessing, and Estimation (project-dependent)
5. Bioinformatics Analysis
6. Raw Data Files
Related Services
Protein Disulfide Bonds Identification and Quantitative Analysis Service
How to order?







