Glycan Sulfation Modification Analysis Service
- Serum / Plasma
- Cell culture supernatants
- Tissue homogenates
- Purified glycoprotein or enriched fractions
Glycan sulfation modification analysis refers to the process of identifying and analyzing sulfate group modifications on glycan molecules. Glycan sulfation refers to the addition of sulfate groups (SO₃²⁻) to glycans attached to proteins or lipids, representing a critical post-translational modification in glycobiology. This modification regulates essential biological processes including cell signaling, immune modulation, and development. Aberrant glycan sulfation is closely associated with cancer, diabetes, and neurodegenerative diseases.
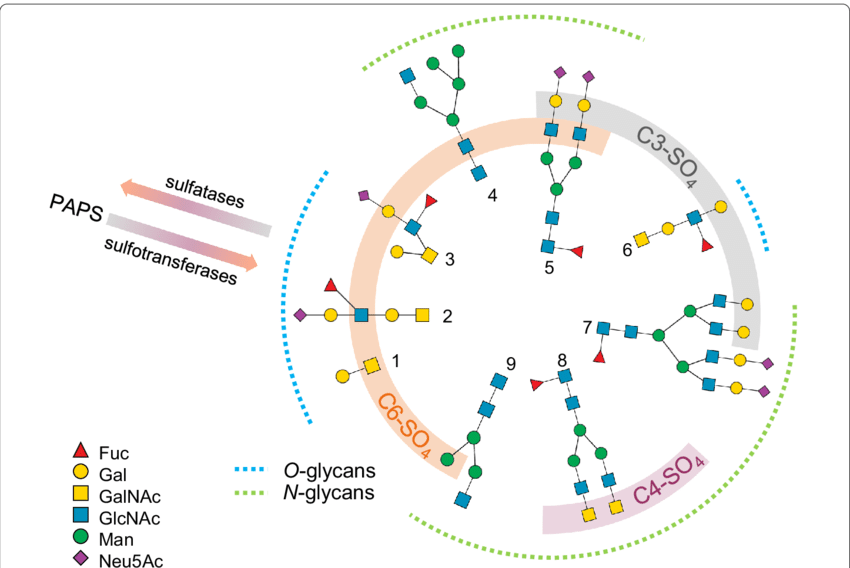
Chuzel, L. et al. Microb Cell Fact. 2021.
Figure 1. N- and O-glycan Sulfation
MtoZ Biolabs offers high-resolution Glycan Sulfation Modification Analysis Service that utilizes Thermo Fisher Q Exactive HF and Orbitrap Fusion Lumos mass spectrometers coupled with Nano-LC platforms. Our Glycan Sulfation Modification Analysis Service enables precise identification of sulfation types, site localization, and structural variation of sulfated glycans, supporting research in disease mechanisms, therapeutic glycoprotein characterization, and glycoengineering.
Analysis Workflow
MtoZ Biolabs offers specialized Glycan Sulfation Modification Analysis Service that accurately identifies sulfation sites, quantifies modifications, reveals structural patterns, and assesses molecular heterogeneity. With advanced mass spectrometry and integrated analytical platforms, we provide comprehensive support throughout the entire process, from sample preparation to data interpretation, ensuring reliable and high-quality results for a wide range of research applications.
1. Sample Preparation
Tailored to sample type (e.g., glycoproteins, glycolipids), ensuring removal of interfering substances and optimization for mass spectrometry compatibility.
2. Sulfated Glycan Enrichment
Selective enrichment methods such as anion exchange chromatography and sulfate-affinity capture enhance detection of low-abundance sulfated species.
3. High-Resolution Mass Spectrometry
LC–MS/MS: Comprehensive mapping of sulfation sites and glycan structures.
MALDI-TOF-MS: Rapid molecular weight profiling.
CE-MS: Charge-based separation of sulfated isoforms.
HPLC-UV/FLD: Targeted quantification via UV or fluorescence detection.
4. Bioinformatics Analysis & Report Delivery
Automated pipelines using Byonic and Proteome Discoverer for site identification, modification pattern recognition, and quantification. Deliverables include site tables, abundance comparisons, structural isomer profiles, and interpretation.
Why Choose MtoZ Biolabs?
✅ High-Precision Analytical Platform: Utilizing Orbitrap-based MS with sub-ppm mass accuracy to achieve site-specific characterization of sulfation, including analysis of multiply sulfated glycans.
✅ Broad Sample Compatibility: Accepts human, animal, or plant-derived materials in various formats: serum, plasma, tissue homogenates, cell culture supernatants, or purified glycoproteins.
✅ Structural and Functional Insight: Capable of determining the exact location and combination of sulfate groups on individual glycans, supporting biological interpretation in complex matrices.
✅ Integrated Bioinformatics Support: Full-spectrum data processing and functional annotation help elucidate the biological relevance of sulfated glycan patterns.
✅ Customizable Analysis Options: Flexible service packages tailored to basic research, clinical discovery, or biopharmaceutical quality control.
Sample Submission Suggestions
Our Glycan Sulfation Modification Analysis Service accepts a wide variety of sample types including:
Sample volume and preparation requirements vary depending on the sample matrix and analysis goals. Please contact our technical team before submission to receive a customized sample preparation guide and optimize analytical outcomes.
Applications
Our Glycan Sulfation Modification Analysis Service is applicable to a wide range of biological and therapeutic research areas:
· Disease Biomarker Discovery: Identify sulfated glycan profiles linked to cancer, autoimmune diseases, or inflammation.
· Biopharmaceutical Quality Control: Monitor sulfation levels in therapeutic glycoproteins to ensure consistency and regulatory compliance.
· Cell Signaling & Development Studies: Elucidate roles of glycan sulfation in cell differentiation, adhesion, and communication.
· Vaccine and Glycoengineering Research: Design sulfated glycan structures for enhanced immune activation or drug targeting.
· Targeted Drug Discovery: Map sulfated glycan distribution and identify actionable targets for small-molecule intervention.
By choosing MtoZ Biolabs, you gain access to cutting-edge instrumentation, expert data interpretation, and fully customizable workflows tailored to your project goals. Our Glycan Sulfation Modification Analysis Service empowers researchers in glycobiology, disease mechanism exploration, and biopharmaceutical development with reliable, publication-ready results.
Contact us today for a consultation or to receive a tailored service proposal.
FAQ
Q1: Can Orbitrap systems resolve glycans with multiple sulfate groups?
Yes. The Orbitrap Fusion Lumos MS system provides exceptional mass accuracy and dynamic range, making it ideal for resolving multiply sulfated glycans. With HCD or ETD fragmentation modes, we can precisely locate sulfate groups and mitigate fragmentation loss.
Q2: What quantification methods do you offer for sulfated glycan analysis?
MtoZ Biolabs employs a range of quantification strategies based on project needs, including label-based methods such as TMT and SILAC via LC–MS/MS, label-free quantification, and fluorescence or UV detection coupled with HPLC platforms. All methods are supported by internal standard calibration and standard curve validation to ensure accuracy, reproducibility, and cross-sample comparability.
What Could be Included in the Report?
1. Comprehensive Experimental Details
2. Materials, Instruments, and Methods
3. Total Ion Chromatogram & Quality Control Assessment
4. Data Analysis, Preprocessing, and Estimation
5. Bioinformatics Analysis
6. Raw Data Files
Related Services
Glycan Modification Analysis Service
Glycan Acetylation Modification Analysis Service
How to order?







