SEC Peptide Purity Analysis Service
- Peptide Drug Development
- Vaccine Design
- Formulation Process Optimization
- Functional Peptide Research
- Bioactive Peptide Screening
SEC Peptide Purity Analysis Service is a non-denaturing purity testing service based on molecular size separation, which can evaluate the structural integrity and aggregation state of peptide samples. It is applicable to multiple fields such as peptide drug development, synthetic peptide quality control, and purity verification of vaccines and diagnostic reagents, especially in applications that require accurate identification of monomeric peptides and their aggregates or degradation products.
In peptide research and industrial applications, purity is not only essential for biological activity and stability but also a critical determinant of safety and efficacy. Conventional methods such as reverse-phase HPLC rely on hydrophobicity-based separation and may not accurately characterize molecular aggregation or conformational changes. Size Exclusion Chromatography (SEC) separates molecules based on size differences within a porous stationary phase, allowing identification and quantification of monomers, oligomers, and high-molecular-weight impurities under native conditions. This provides purity information that is more structurally relevant.
Leveraging the SEC platform, MtoZ Biolabs offers SEC Peptide Purity Analysis Service for highly sensitive separation and quantification of monomeric peptides, aggregates, and high-molecular-weight impurities in various peptide samples. This service enables rapid and accurate analysis, supporting researchers and industrial clients in acquiring critical quality attribute (CQA) data.
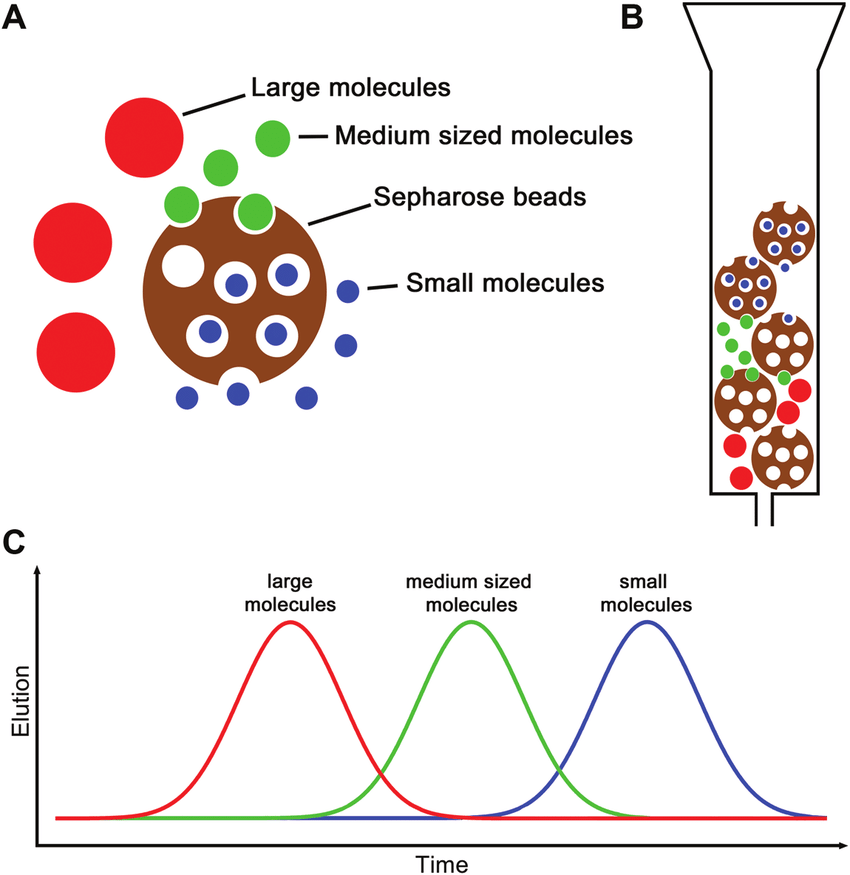
Ludwig N. et al. Curr Protoc Immunol. 2019.
Figure 1. Illustration of the SEC principle.
Analysis Workflow
The general analysis workflow of SEC Peptide Purity Analysis Service is as follows:
1. Sample Preparation
Dissolve the peptide sample in a compatible buffer, filter to remove particulate impurities, and ensure system compatibility and detection stability.
2. Method Setup
Select an appropriate chromatographic column and mobile phase system based on peptide size and analytical objectives; set parameters such as column temperature and flow rate.
3. Chromatographic Separation
Separate monomers, oligomers, and impurity components in the peptide sample under non-denaturing conditions using size exclusion chromatography (SEC).
4. Detection and Recording
Use a detector to record the chromatogram and monitor the elution time and peak area of each component.
5. Data Analysis
Analyze peak area ratios to assess purity, aggregate content, and potential degradation products, and generate a standardized analytical report.
Service Advantages
Advanced Analysis Platform: MtoZ Biolabs established an advanced SEC Peptide Purity Analysis Service platform, guaranteeing reliable, fast, and highly accurate analysis service.
One-Time-Charge: Our pricing is transparent, no hidden fees or additional costs.
Non-denaturing Analysis: SEC operates under mild conditions without requiring denaturants or extreme pH treatments, making it suitable for analyzing peptides in their native states.
Compatibility with Multiple Detectors: The method supports signal acquisition using UV detectors, refractive index detectors, or fluorescence detectors, enabling multidimensional analysis.
Broad Sample Compatibility: Applicable to various types of peptide samples, including synthetic peptides, natural peptides, recombinant peptides, and vaccine-related peptides.
Sample Submission Suggestions
Sample Types: Accepts various peptide forms, including synthetic peptides, natural peptides, recombinant peptides, and peptide-based drug formulations.
Storage and Transportation: It is recommended to store at low temperature (−20°C or −80°C), transport on dry ice and avoid repeated freezing and thawing.
Additional Notes: We recommend contacting us prior to sample submission for detailed and tailored sample preparation guidelines.
Applications
Application examples of SEC Peptide Purity Analysis Service:
Evaluate polymer content in active pharmaceutical ingredients or formulations to ensure product quality and safety.
Assess the aggregation state of synthetic peptides to validate their immunogenic potential.
Monitor the impact of purification and storage conditions on peptide purity and aggregation.
Analyze structural changes in bioactive peptides under different treatment conditions.
Determine the purity of peptides extracted from natural sources or fermentation products to support subsequent activity validation and functional studies.
Deliverables
1. Comprehensive Experimental Details
2. Materials, Instruments, and Methods
3. Total Ion Chromatogram & Quality Control Assessment (project-dependent)
4. Data Analysis, Preprocessing, and Estimation (project-dependent)
5. Raw Data Files
* For research use only. Not for diagnostic purposes. Samples from individual customers or for personal use are not accepted.
Related Services
Peptide Purity Analysis Service
How to order?







