RP-HPLC Peptide Purity Analysis Service
- Peptide Drug Development and Quality Control
- Synthetic Peptide Quality Assessment
- Proteomics Research
- Vaccine Development and Antibody Research
RP-HPLC Peptide Purity Analysis Service refers to a service that utilizes reverse-phase chromatography columns and gradient elution systems to separate and quantify target components and impurities in peptide samples based on retention time and peak area. This service can effectively evaluate the main peak purity of peptides, identify impurity types such as synthetic by-products, oxidation products, and incomplete deprotection, and provide key data support for the development, quality control, and drug application of peptide products.
Peptide purity directly affects biological activity, stability, and safety. Therefore, accurate purity analysis is an essential step in peptide drug development, functional peptide research, and quality control. Reverse-Phase High-Performance Liquid Chromatography (RP-HPLC) is one of the most widely used and reliable techniques for analyzing peptide compound purity. During RP-HPLC analysis, peptides in the sample are separated sequentially based on their retention times on the chromatography column and detected by a UV detector. By interpreting the chromatogram, researchers can precisely assess peptide purity, identify potential impurities, and calculate the proportion of the main component.
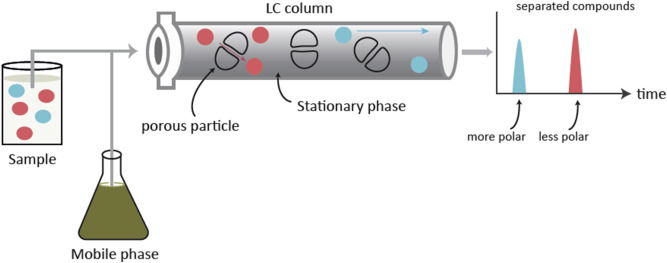
Alsaleh M. et al. J Clin Exp Hepatol. 2019.
Figure 1. Separation in reversed-phase liquid chromatography.
Leveraging a high-performance RP-HPLC platform, MtoZ Biolabs offers RP-HPLC Peptide Purity Analysis Service to accurately evaluate the main peak purity and impurity profile of peptide samples. This service provides reliable data support for peptide drug development, functional peptide screening, and structural modification studies. It is suitable for a wide range of research and production scenarios, including peptide synthesis, purification, and quality control.
Analysis Workflow
The general workflow of RP-HPLC Peptide Purity Analysis Service is as follows:
1. Sample Preprocessing
Dissolve the peptide sample in an appropriate solvent; if necessary, perform centrifugation, filtration, or desalting to remove particulates and interfering substances.
2. Method Setup and System Preparation
Set chromatographic conditions based on the properties of the peptide, including column type, mobile phase composition, gradient program, and detection wavelength.
3. Sample Injection and Separation Analysis
Inject the prepared sample into the RP-HPLC system. Peptides and impurities are separated using a reverse-phase column, and chromatograms are recorded in real time.
4. Data Acquisition and Purity Calculation
Calculate the relative purity of the target peptide based on the peak area of the main peak and impurity peaks in the chromatogram. Identify potential impurity types.
5. Result Output and Report Generation
Compile and summarize the analysis results into a complete report, including chromatograms, purity percentage, analytical conditions, and experimental notes for client reference and quality evaluation.
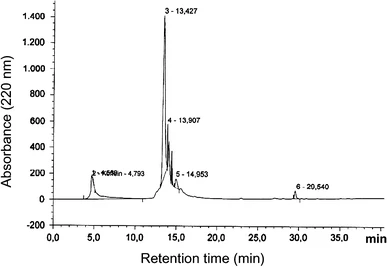
Murariu, M. et al. Int J Pept Res Ther. 2009.
Figure 2. RP-HPLC chromatogram of peptide P2.
Service Advantages
1. Advanced Analysis Platform: MtoZ Biolabs established an advanced RP-HPLC Peptide Purity Analysis Service platform, guaranteeing reliable, fast, and highly accurate analysis service.
2. One-Time-Charge: Our pricing is transparent, no hidden fees or additional costs.
3. Broad Applicability: Suitable for various types of peptides, including synthetic peptides, natural peptides, and modified peptides, meeting the needs of both research and production.
4. Customized Service: Offers personalized analytical plan design to help clients tailor experimental parameters based on specific requirements, ensuring result accuracy and scientific reliability.
Sample Submission Suggestions
Sample Types: Supports synthetic peptides and peptides derived from natural sources.
Storage and Shipping: Store samples at low temperature (−80 °C) in either lyophilized or solution form. Shipping on dry ice is recommended. Avoid repeated freeze–thaw cycles.
Additional Notes: We recommend contacting us prior to sample submission for detailed and tailored sample preparation guidelines.
Applications
Application examples of RP-HPLC Peptide Purity Analysis Service:
Evaluate the purity of candidate peptide drugs to ensure consistency and stability of active pharmaceutical ingredients.
Assess the quality of synthetic peptides by identifying unreacted impurities or degradation products from the synthesis process.
Measure the purity of peptide samples to support downstream proteomics analyses.
Perform purity control on vaccine candidate peptides to ensure their immunogenicity and therapeutic efficacy.
Deliverables
1. Comprehensive Experimental Details
2. Materials, Instruments, and Methods
3. Total Ion Chromatogram & Quality Control Assessment (project-dependent)
4. Data Analysis, Preprocessing, and Estimation (project-dependent)
5. Raw Data Files
* For research use only. Not for diagnostic purposes. Samples from individual customers or for personal use are not accepted.
Related Services
Peptide Purity Analysis Service
How to order?







