Peptidomics-Based Drug Side Effects Research Service
- Tissues (e.g., liver, brain, kidney)
- Cultured cells (adherent or suspension)
- Biofluids (e.g., plasma, serum, cerebrospinal fluid)
- Tissues: ≥ 200 mg
- Biofluids: ≥ 200 µL
- Cells: ≥ 1 × 10⁷ cells per sample
- Samples should be stored at -80℃ and shipped with dry ice.
The development of new drugs is an intricate process that requires not only the evaluation of therapeutic efficacy but also a thorough understanding of potential side effects that could compromise patient safety. Side effects may arise from various factors, including unintended interactions with non-target proteins, alterations in cellular signaling pathways, or disturbances in homeostasis. These adverse effects can lead to reduced drug effectiveness, unwanted toxicity, and, ultimately, clinical failure. As drug development advances, it is crucial to identify and mitigate these side effects early in the process to ensure a safer, more effective therapeutic outcome.
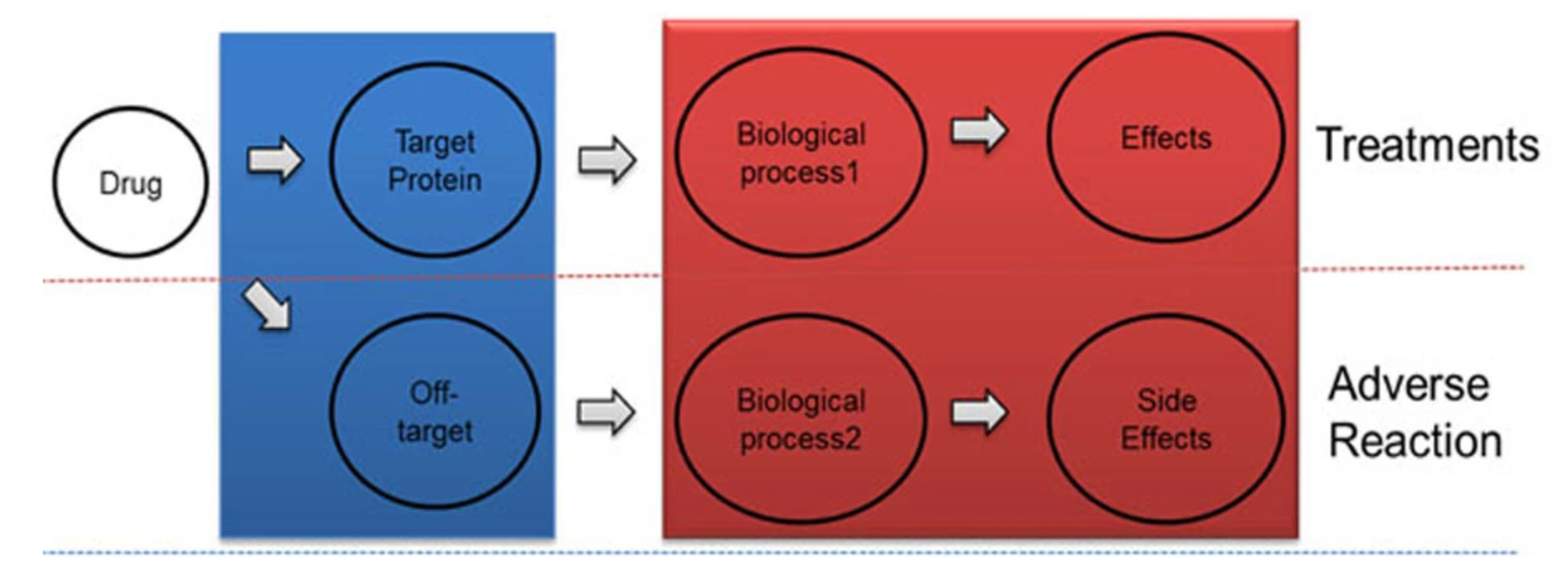
Figure1. Flow of Drug Treatments and Adverse Reaction
Peptidomics, the study of the entire set of peptides expressed in a biological sample, offers a powerful tool for detecting side effects induced by drug treatments. Peptides play a critical role in regulating physiological processes such as signal transduction, immune responses, metabolic pathways, and cellular communication. They are often the first molecules to respond to changes in cellular function and thus serve as sensitive biomarkers for adverse drug reactions. Unlike traditional approaches that focus on broad-scale proteomics or genomics, peptidomics provides a more targeted analysis of bioactive peptides. Using high-resolution mass spectrometry, we can identify and quantify peptides with high sensitivity, uncovering subtle changes that occur as a result of drug exposure. This allows us to assess alterations in peptide expression, post-translational modifications, and peptide-mediated signaling events that may indicate early signs of toxicity or other unwanted effects.
Service at MtoZ Biolabs
MtoZ Biolabs offers a full-spectrum Peptidomics-Based Drug Side Effects Research Service tailored to reveal drug-related adverse effects through the lens of peptide dynamics. Leveraging high-resolution LC-MS/MS, advanced peptide enrichment protocols, and expert bioinformatics support, we empower clients to investigate side effect mechanisms, identify biomarkers, and optimize drug candidates across a range of therapeutic areas. Our key service offerings include:
💠Mechanistic Insight into Side Effects
We analyze drug-induced peptide alterations to reveal proteolytic events and signaling disruptions underlying organ toxicity, inflammation, or cellular stress.
💠Biomarker Identification
By comparing peptidomic profiles of treated versus control samples, we identify specific peptides associated with adverse drug responses. These peptide signatures can serve as early biomarkers for organ-specific toxicity, neurotoxicity, or systemic stress, providing valuable tools for preclinical screening and risk assessment.
💠Pharmacokinetic Studies
We offer time-course analysis of peptidome changes in relation to drug administration, supporting pharmacokinetic (PK) studies. By correlating peptide fluctuations with drug exposure, we help delineate dose-response relationships, metabolic stability, and potential time-dependent toxicities, enhancing the understanding of drug kinetics at the molecular level.
💠Drug Candidate Optimization
Our peptidomics platform aids in comparing structurally related compounds or formulation variants, helping clients identify lead candidates with favorable safety profiles. By pinpointing off-target effects or unexpected proteolytic consequences, we provide data to guide structural refinements or dosing strategies.
💠Adverse Reaction Monitoring
We support both animal studies and cell-based assays for real-time monitoring of drug-induced peptidomic changes. This enables comprehensive surveillance of potential side effects in various biological systems and can be used to evaluate acute toxicity, chronic exposure, or combined drug regimens.
Analysis Workflow
1. Sample Preparation: Biological samples are processed to extract endogenous peptides using optimized precipitation and filtration protocols.
2. Peptide Enrichment: Peptides are purified via SPE or magnetic bead methods and desalted for MS analysis.
3. LC-MS/MS Analysis: High-resolution mass spectrometry is used for peptide identification and quantification.
4. Data Processing: Peptide spectra are analyzed using advanced software to identify differentially expressed peptides.
5. Bioinformatics Interpretation: Identified peptides are mapped to proteins, pathways, and protease activity to reveal drug-induced changes and side effect mechanisms.
Why Choose MtoZ Biolabs?
✔️Expertise in Peptidomics and Drug Safety
Our team combines deep experience in peptide science, mass spectrometry, and toxicology to deliver accurate and insightful results.
✔️Cutting-Edge Analytical Platforms
MtoZ Biolabs established an advanced Peptidomics-Based Drug Side Effects Research Service platform, guaranteeing reliable, fast, and highly accurate analysis service.
✔️High-Data-Quality
Deep data coverage with strict data quality control. AI-powered bioinformatics platform integrates all peptidomics-based drug side effects research data, providing clients with a comprehensive data report.
✔️Flexible Project Design
We offer fully customized workflows adapted to your compound type, research model, and study goals, whether for early discovery or preclinical validation.
✔️One-Time-Charge
Our pricing is transparent, no hidden fees or additional costs.
Sample Submission Suggestions
1. Sample Types
We accept various sample types, including but not limited to:
2. Sample Quantity
3. Storage and Transport
*Note: If you have special sample types or require additional guidance, please contact us for personalized support before shipping.
Deliverables
1. Comprehensive Experimental Details
2. Materials, Instruments, and Methods
3. Total Ion Chromatogram & Quality Control Assessment
4. Data Analysis, Preprocessing, and Estimation
5. Bioinformatics Analysis
6. Raw Data Files
To learn more about our Peptidomics-Based Drug Side Effects Research Service, please contact us. Our team of experts is ready to provide customized solutions for your drug development needs.
Related Services
Peptide Mass Spectrometric Identification Service
Peptide Structure Determination Service
How to order?







