Peptide Drug Quality Control Service
- High-Performance Liquid Chromatography (HPLC): for high-resolution peptide purity profiling and impurity separation
- Mass Spectrometry (MS): for accurate mass determination, sequence confirmation, and modification analysis
- Nuclear Magnetic Resonance (NMR) Spectroscopy: for structural integrity and conformational studies
- Amino Acid Analysis: for composition verification and peptide backbone confirmation
- Potency Assays: for biological activity and dose-response measurements
- Endotoxin Detection: for safety assurance in therapeutic applications
- Synthetic or recombinant peptide drugs
- Peptide formulations (lyophilized or in solution)
- Crude or purified peptide products
- For HPLC and MS analysis: ≥ 0.5 mg
- For structural analysis (NMR, amino acid analysis): ≥ 1–2 mg
- For endotoxin testing: ≥ 0.5 mL of solution at testing concentration
- For potency assays: as required based on assay type (please consult in advance)
- Samples should be stored at -80℃ and shipped with dry ice
Peptide-based drugs have become an increasingly significant class of therapeutics due to their high specificity and low toxicity. With advances in peptide drug development, there is a growing need for stringent quality control processes to ensure the safety, efficacy, and consistency of peptide-based therapies. The complex nature of peptides, which includes structural variations, post-translational modifications (PTMs), and issues such as aggregation and stability, requires specialized analytical techniques to evaluate their quality comprehensively.
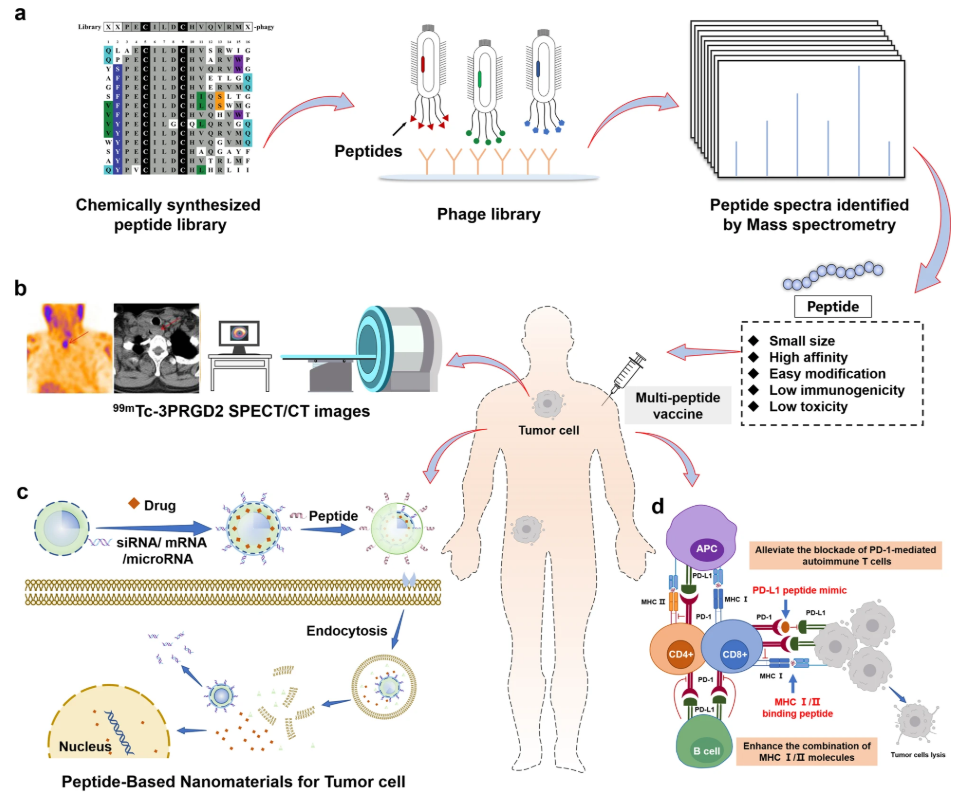
Figure 1. Application of Peptides in Tumor Therapy
MtoZ Biolabs offers a Peptide Drug Quality Control Service that uses advanced mass spectrometry and analytical techniques to assess peptide purity, stability, aggregation, PTMs, and degradation. Our service provides the critical data necessary to confirm the quality and consistency of peptide-based drugs, ensuring they meet regulatory standards and are safe for clinical use.
Service at MtoZ Biolabs
MtoZ Biolabs provides a full suite of analytical services to support the quality control of peptide-based drugs. Our platform integrates advanced instrumentation, validated protocols, and experienced scientific staff to ensure your peptide therapeutics meet the highest standards of safety, efficacy, and regulatory compliance. The services we provide include:
We evaluate the purity of peptide products using high-performance liquid chromatography (HPLC) and mass spectrometry (MS), ensuring the absence of impurities, by-products, and degradation fragments.
Using mass spectrometry (MS) and nuclear magnetic resonance (NMR), we confirm the identity and molecular structure of peptides, verifying sequence accuracy and confirming batch-to-batch consistency.
💠 Peptide Drug Potency Evaluation
We perform bioactivity and potency assays to assess the functional effectiveness of peptide drugs, providing critical data for preclinical and regulatory submissions.
💠 Peptide Drug Structural Integrity Analysis
Structural analysis using NMR, MS, and amino acid analysis ensures the correct folding, sequence fidelity, and chemical integrity of peptide drugs throughout development and storage.
💠 Endotoxin Testing for Peptide Drugs
We conduct endotoxin detection using validated Limulus Amebocyte Lysate (LAL) assays to confirm the safety of peptide products intended for in vivo or clinical use.
Our analytical approaches include:
By combining these technologies, MtoZ Biolabs delivers comprehensive, precise, and reliable quality control solutions for peptide drug research, development, and production.
Why Choose MtoZ Biolabs?
☑️Comprehensive Analytical Coverage: We offer end-to-end quality control services for peptide drugs, including purity testing, identity verification, potency evaluation, structural analysis, and endotoxin detection.
☑️Advanced Analysis Platform: MtoZ Biolabs established an advanced Peptide Drug Quality Control Service platform, guaranteeing reliable, fast, and highly accurate analysis service.
☑️Expert Team and Proven Experience: Our scientific team has extensive experience in peptide analysis, regulatory compliance, and drug characterization, supporting clients from early-stage research through product release.
☑️Tailored Solutions: We provide flexible service packages customized to your molecule type, formulation, and development stage, ensuring efficiency and relevance to your project goals.
☑️One-Time-Charge: Our pricing is transparent, no hidden fees or additional costs.
Applications
1. Pharmaceutical Development
Our service is critical for the development of peptide-based drugs. By ensuring that peptides meet quality standards for purity, stability, and functionality, we help developers produce reliable and effective therapeutics.
2. Biologics Manufacturing
For companies involved in the large-scale manufacturing of peptide drugs, our quality control service ensures that each batch produced is consistent and free from impurities, guaranteeing patient safety.
3. Biotech and Research
Researchers developing peptide-based diagnostics or therapeutic agents rely on our service to ensure that their experimental drugs maintain high purity and stability for preclinical and clinical studies.
4. Peptide Formulation and Storage
Understanding the behavior of peptides under different storage conditions is critical for ensuring long-term stability. Our service evaluates peptide stability under varying conditions, helping optimize storage and formulation.
Sample Submission Suggestions
1. Accepted Sample Types
2. Recommended Sample Amount
3. Storage and Shipping
*Note: If you have special sample types or require additional guidance, please contact our technical team for personalized support before shipping.
What Could be Included in the Report?
1. Comprehensive Experimental Details
2. Materials, Instruments, and Methods
3. Total Ion Chromatogram & Quality Control Assessment
4. Data Analysis, Preprocessing, and Estimation
5. Bioinformatics Analysis
6. Raw Data Files
Related Services
How to order?







