Peptide Disulfide Bond and Free Cysteine Detection Service
- Peptide Drug Quality Control: Used for the structural identification and stability study of peptide drugs, ensuring the correct folding of disulfide bonds and the controllability of free cysteine.
- Vaccine Research and Immunology: Analyzes disulfide bonds and free cysteine in immunopeptides or antigenic peptides to optimize their folding and immune activation capabilities.
- Peptide-Ligand/Metal Interaction: Investigates the binding mechanisms of peptides with small molecules or metal ions, exploring the role of disulfide bonds and free cysteine in molecular recognition.
- Natural Bioactive Peptide Screening: Evaluates the stability of disulfide bonds and the concentration of free cysteine in naturally extracted peptides, advancing the development and optimization of functional peptides.
Peptide Disulfide Bond and Free Cysteine Detection Service is a specialized service that uses a range of analytical techniques to detect disulfide bonds and free cysteine residues in peptide molecules. This service is designed to assist researchers in identifying and quantifying the formation and cleavage of disulfide bonds, as well as the concentration changes of free cysteine, thereby revealing the peptide's stability, folding state, and biological activity.
The stability, folding, and biological activity of peptides are often regulated by their three-dimensional structure, with the formation of disulfide bonds and the status of free cysteine residues being critical to peptide function. Disulfide bonds are structures formed by the covalent linkage of sulfur atoms from two cysteine residues and are widely present in natural peptides and proteins. The content and position of disulfide bonds and free cysteine may influence peptide folding and biological activity, and disulfide bonds can undergo reduction reactions, while free cysteine may be oxidized or reduced under changing environmental conditions. Effectively detecting these structural changes is crucial for peptide drug development, structural biology research, and studies of related functions.
Leveraging platforms such as mass spectrometry, chromatography, and circular dichroism, MtoZ Biolabs provides Peptide Disulfide Bond and Free Cysteine Detection Service to comprehensively analyze disulfide bonds and free cysteine in peptide molecules, helping to reveal the connection status of disulfide bonds in peptides, the concentration of free cysteine and its potential impact on peptide structure and biological activity.
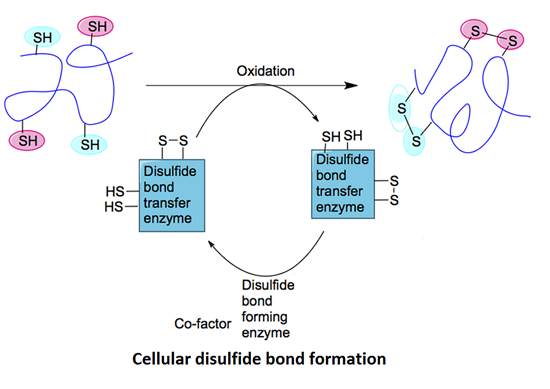
Patil NA. et al. Int J Mol Sci. 2015.
Analysis Workflow
The general analysis process for Peptide Disulfide Bond and Free Cysteine Detection Service is as follows:
1. Sample Preparation
Perform an initial inspection of the peptide sample to ensure its purity meets the requirements. The sample concentration and solvent conditions will be adjusted according to the experimental requirements.
2. Analysis Method Selection
Select the appropriate detection technique based on the characteristics of the peptide. Common methods include mass spectrometry (MS) analysis, liquid chromatography (LC) analysis, and circular dichroism (CD) analysis to ensure comprehensive detection of disulfide bonds and free cysteine.
3. Data Acquisition
Use mass spectrometry (MS) to analyze the disulfide bond cleavage products of the peptide, liquid chromatography for quantitative analysis, and CD spectroscopy to assess the reduction of disulfide bonds and changes in the peptide's secondary structure.
4. Data Analysis and Interpretation
Combine the experimental data to thoroughly interpret the disulfide bond connectivity and the content of free cysteine residues in the peptide.
5. Report Generation
Based on the data analysis, generate a detailed report that includes qualitative and quantitative information about disulfide bonds, the concentration of free cysteine, and changes in the peptide's secondary structure.
Service Advantages
Advanced Analysis Platform: MtoZ Biolabs established an advanced Peptide Disulfide Bond and Free Cysteine Detection Service platform, guaranteeing reliable, fast, and highly accurate analysis service.
One-Time-Charge: Our pricing is transparent, no hidden fees or additional costs.
Combination of Multiple Techniques: A comprehensive analysis of disulfide bonds and free cysteine in peptides is performed by combining mass spectrometry (MS), circular dichroism (CD), redox titration, and other techniques.
Customized Solutions: Flexible experimental designs and personalized analysis plans are provided based on the characteristics of the peptide and the research needs, catering to different technical requirements of the clients.
Sample Submission Suggestions
Sample Types: Supports synthetic peptides, naturally sourced peptides, recombinant expressed peptides, etc.
Storage and Transportation: Store at low temperatures (−20°C or −80°C). Dry ice transportation is recommended to avoid repeated freeze-thaw cycles.
Additional Notes: We recommend contacting us prior to sample submission for detailed and tailored sample preparation guidelines.
Applications
Examples of applications for Peptide Disulfide Bond and Free Cysteine Detection Service:
Deliverables
1. Comprehensive Experimental Details
2. Materials, Instruments, and Methods
3. Total Ion Chromatogram & Quality Control Assessment (project-dependent)
4. Data Analysis, Preprocessing, and Estimation (project-dependent)
5. Bioinformatics Analysis
6. Raw Data Files
Related Services
Disulfide Bond Analysis Services
How to order?







