MS Peptide Purity Analysis Service
- Peptide Drug Development and Quality Control
- Synthetic Peptide Validation and Process Optimization
- Vaccine Development and Antigen Design
MS Peptide Purity Analysis Service is a peptide purity testing service based on a high-resolution mass spectrometry platform, which aims to accurately evaluate the main components and potential impurities in synthetic or natural peptide samples. This service makes up for the shortcomings of traditional chromatographic analysis methods in impurity identification and low-abundance component detection, and is particularly suitable for quality control and R&D verification of products such as bioactive peptides, diagnostic peptides, and vaccine peptides that have high purity requirements.
In peptide drug development, functional peptide screening, and synthetic peptide research, peptide purity directly impacts biological activity, stability, and experimental reproducibility. Traditional purity analysis methods such as high-performance liquid chromatography (HPLC) offer overall purity assessment but often fall short in accurately identifying complex impurities. MS Peptide Purity Analysis, a mass spectrometry-based approach, enables the direct detection of all ion signals within a peptide sample using high-resolution mass spectrometry platforms, allowing rapid identification of major components and trace impurities. This provides a more sensitive and comprehensive quality control solution for both synthetic and natural peptides.
Based on the advanced mass spectrometry platform, MtoZ Biolabs provides MS Peptide Purity Analysis Service to accurately identify the molecular weight and main component information of peptides, and can perform qualitative or quantitative analysis on low-abundance impurities, providing reliable technical support for peptide drug development, biological reagent quality control, functional peptide screening, etc.
Analysis Workflow
The general workflow of MS Peptide Purity Analysis Service is as follows:
1. Sample Preparation
Peptide samples undergo preliminary quality assessment, including concentration measurement, buffer composition check, and necessary dilution or desalting, to ensure compatibility with the mass spectrometry platform.
2. Mass Spectrometry Detection
Perform full-scan analysis using a high-resolution mass spectrometry platform; MS/MS fragmentation analysis may be applied as needed.
3. Data Processing
Use specialized software to extract main ion peaks and impurity peaks, perform peak area integration and spectral comparison, and generate purity and related data.
4. Result Output
Deliver a comprehensive analysis report including spectra, purity results, and impurity descriptions.
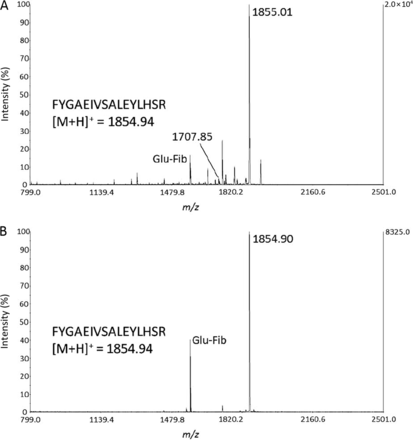
Hoofnagle AN. et al. Clin Chem. 2016.
Figure 1. MALDI-MS spectra of lower-purity (A) and high-purity (B) peptides.
Service Advantages
Advanced Analysis Platform: MtoZ Biolabs established an advanced MS Peptide Purity Analysis Service platform, guaranteeing reliable, fast, and highly accurate analysis service.
One-Time-Charge: Our pricing is transparent, no hidden fees or additional costs.
High-Data-Quality: Deep data coverage with strict data quality control. AI-powered bioinformatics platform integrates all MS Peptide Purity Analysis data, providing clients with a comprehensive data report.
High Structural Specificity: MS/MS fragmentation patterns can be used to further identify the structural information of suspected impurities, effectively distinguishing isomers with similar or identical molecular weights, thereby improving analytical accuracy and reliability.
Broad Sample Compatibility: Supports peptide purity analysis from trace-level samples (as low as pmol scale) to standard mg-scale quantities, covering various research and development stages from initial synthesis screening to final quality control.
Sample Submission Suggestions
Sample Types: Applicable to a wide range of peptide sources, including synthetic peptides, naturally derived peptides, modified peptides, and specific peptide fragments from protein digestion products.
Storage and Shipping: Samples should be stored frozen at −20 °C or below. During transport, the use of dry ice or cold packs is recommended to prevent degradation caused by repeated freeze–thaw cycles.
Additional Notes: We recommend contacting us prior to sample submission for detailed and tailored sample preparation guidelines.
Applications
Application examples of MS Peptide Purity Analysis Service:
Enables quality assessment of candidate peptide drugs by accurately identifying synthetic by-products, degradation products, and unblocked peptides, ensuring drug safety and consistency.
Confirms the molecular weight and composition of target peptides through high-sensitivity mass spectrometry, supporting the optimization of synthetic workflows.
In peptide-based vaccine development, MS purity analysis helps verify the structural integrity and purity of epitope peptides, ensuring the specificity and effectiveness of immune responses.
Deliverables
1. Comprehensive Experimental Details
2. Materials, Instruments, and Methods
3. Total Ion Chromatogram & Quality Control Assessment (project-dependent)
4. Data Analysis, Preprocessing, and Estimation (project-dependent)
5. Raw Data Files
* For research use only. Not for diagnostic purposes. Samples from individual customers or for personal use are not accepted.
Related Services
Peptide Purity Analysis Service
How to order?







