DNA/RNA Drug Structural Characterization Service
- Efficacy and Safety Studies: Confirm the impact of structural features on drug activity and toxicity.
- Formulation Development and Delivery System Optimization: Evaluate the influence of structure on drug stability, aggregation tendency, and delivery efficiency, providing key evidence for formulation development and delivery system optimization.
- Drug Development and Candidate Screening: Verify whether the sequence and modifications of nucleic acid drugs are consistent with design through comprehensive structural analysis, ensuring the accuracy and stability of candidate molecules.
- Quality Control and Consistency Evaluation: Monitor structural integrity and batch-to-batch consistency of drugs during production.
DNA/RNA Drug Structural Characterization Service is a specialized analytical service for the systematic analysis and confirmation of the sequence, secondary structure, and higher-order conformation of DNA/RNA drugs. This service verifies the consistency of the drug with the designed sequence, detects potential modification losses, degradation products, and conformational changes, and provides scientific and reliable structural information to support drug development, quality control, formulation optimization, and regulatory submission.
The stability, delivery efficiency, biological activity, and safety of nucleic acid drugs often directly depend on their molecular modifications and spatial conformation, while structural abnormalities may result in reduced efficacy or safety concerns. Therefore, structural characterization of DNA/RNA drugs is not only a critical step during research and development but also an essential requirement for quality consistency studies and regulatory approval. Through scientific and systematic structural characterization, research risks can be effectively reduced, and the clinical translation and market application of nucleic acid drugs can be accelerated.
Services at MtoZ Biolabs
MtoZ Biolabs provides DNA/RNA Drug Structural Characterization Service covering multiple dimensions from sequence analysis to higher-order structural characterization, helping to achieve an in-depth understanding of the molecular properties and conformational behavior of nucleic acid drugs. We deliver high-resolution, reproducible, and regulatory-compliant structural characterization results to accelerate the development and clinical translation of nucleic acid drugs.
Sequence Analysis
Using highly sensitive nucleotide sequencing and mass spectrometry, we precisely verify the base composition and modifications of DNA/RNA drugs and identify potential impurities or truncated products.
Secondary Structure Analysis
Through techniques such as circular dichroism (CD), we characterize local structural features of DNA/RNA drugs, including hairpin structures, loops, and stems.
Higher-Order Structure Analysis
With methods such as nuclear magnetic resonance (NMR), X-ray crystallography, and atomic force microscopy (AFM), we investigate the global folding and long-range interactions of DNA/RNA drugs.
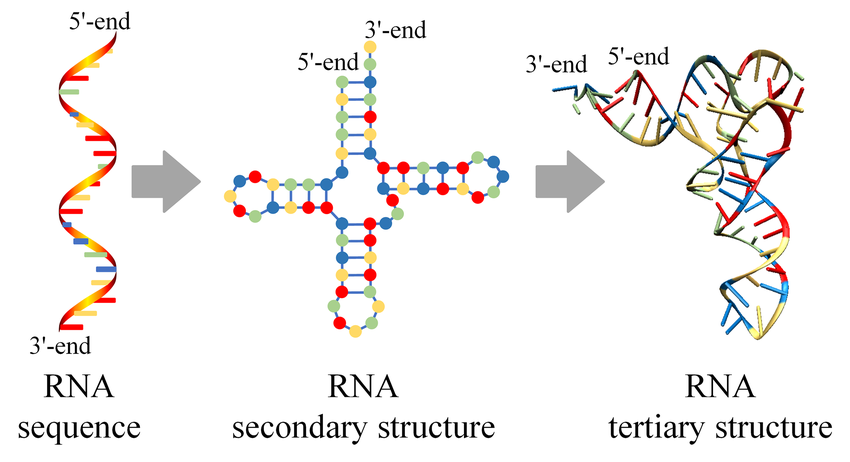
Zhao Q. et al. PLoS Comput Biol. 2021.
Figure 1. RNA primary, secondary, and tertiary structures.
Analysis Workflow
The general workflow of DNA/RNA Drug Structural Characterization Service is as follows:
1. Sample Preparation
Purify DNA/RNA drugs and perform buffer treatment to optimize testing conditions and prevent degradation or conformational changes.
2. Method Selection
Select appropriate combinations of techniques based on research objectives and drug characteristics.
3. Data Acquisition
Use the selected techniques to obtain data on primary sequence, secondary structure, and higher-order conformation.
4. Data Analysis and Interpretation
Integrate experimental data with specialized software and databases, interpret three-dimensional structures, and identify potential structural motifs or interactions.
5. Cross-Validation
Apply different methods to cross-validate results and enhance accuracy and reliability.
6. Report Generation
Provide a complete report including experimental conditions, test results, and quality control data.
Service Advantages
Advanced Analysis Platform: MtoZ Biolabs established an advanced DNA/RNA Drug Structural Characterization Service platform, guaranteeing reliable, fast, and highly accurate analysis service.
One-Time-Charge: Our pricing is transparent, no hidden fees or additional costs.
High-Data-Quality: Deep data coverage with strict data quality control. AI-powered bioinformatics platform integrates all DNA/RNA Drug Structural Characterization data, providing clients with a comprehensive data report.
Customized Solutions: Develop personalized characterization strategies based on drug type and research objectives.
Sample Submission Suggestions
Sample Types
Applicable to various forms of DNA/RNA drugs, including siRNA, mRNA, antisense oligonucleotides, and aptamers.
Storage and Transportation
Recommended storage at -20°C or below, with shipment on dry ice during transportation.
It is recommended to contact the MtoZ Biolabs technical team prior to sample submission to obtain detailed and tailored guidelines for sample preparation and submission.
Applications
FAQ
Q1: What Techniques Are Commonly Used in DNA/RNA Drug Structural Characterization Service?
A1: Common methods include mass spectrometry for molecular weight and modification detection, nuclear magnetic resonance (NMR) and X-ray crystallography for high-resolution three-dimensional structural analysis, circular dichroism (CD) for secondary structure analysis, and atomic force microscopy (AFM) for morphological observation. The combined application of multiple platforms provides comprehensive information from primary sequence to higher-order conformation.
Q2: Can Low-Abundance Degradation Products or Modification Losses Be Detected?
A2: Yes. Mass spectrometry, particularly LC-MS/MS, offers high sensitivity and can detect low-abundance impurities, truncated fragments, and modification losses, thereby supporting the evaluation of drug purity and stability.
Deliverables
1. Comprehensive Experimental Details
2. Materials, Instruments, and Methods
3. Total Ion Chromatogram & Quality Control Assessment (project-dependent)
4. Data Analysis, Preprocessing, and Estimation (project-dependent)
5. Bioinformatics Analysis
6. Raw Data Files
Related Services
DNA/RNA Drug Quantitative Analysis Service
How to order?







