Sucralose Analysis Service | Pharmaceutical Excipient
MtoZ Biolabs provides comprehensive Sucralose Analysis Service covering structure identification, purity and impurity profiling, content determination, physicochemical characterization, and stability evaluation. This service generates scientific data to support pharmaceutical R&D, excipient quality control, and food industry applications. With advanced platforms and experienced scientists, we ensure accurate, reproducible, and regulatory-compliant results.
Sucralose (C₁₂H₁₉Cl₃O₈) is a high-intensity sweetener produced by selective chlorination of sucrose. It appears as a white to off-white crystalline powder with a sweetness about 600 times that of sucrose. Sucralose offers excellent thermal stability, is not metabolized by the human body, and provides zero calories. These features make it a widely used excipient in pharmaceutical formulations such as tablet coatings, as well as a common additive in food and beverages. Comprehensive analysis of sucralose supports consistency across batches, guides formulation optimization, and fulfills regulatory and market access requirements.
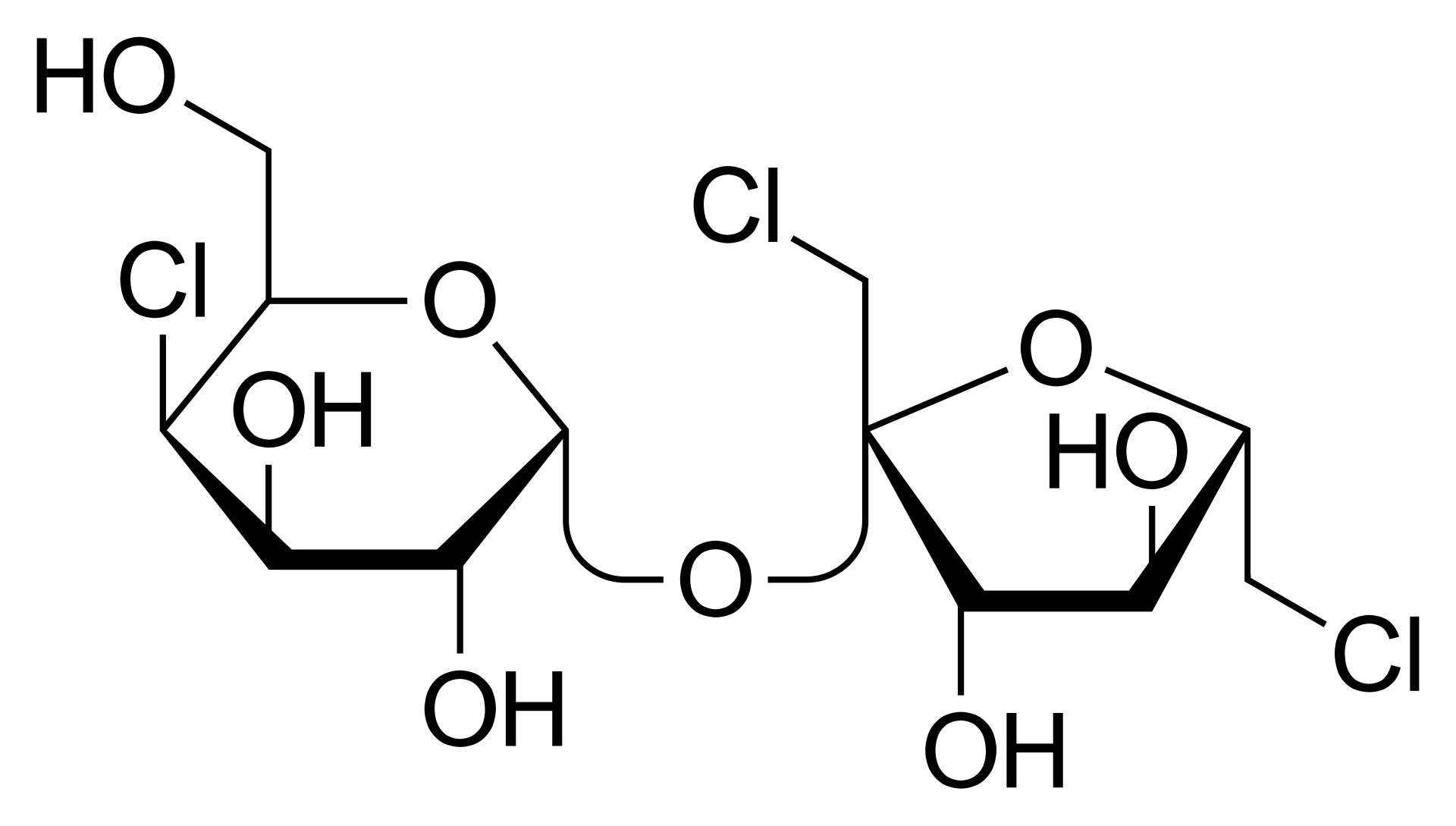
Figure 1. Molecular Structure of Sucralose
Services at MtoZ Biolabs
MtoZ Biolabs offers end-to-end analytical solutions for sucralose, addressing pharmaceutical excipient requirements while also supporting food industry applications:
● Structural Identification
HPLC, FTIR, NMR, and MS confirm the molecular structure and verify consistency with reference standards, ensuring product authenticity.
● Content and Purity Determination
Using HPLC with external or internal standards, we perform quantitative content measurement combined with impurity analysis. These results demonstrate compliance with pharmacopeial and international specifications.
● Specific Rotation Measurement
Polarimetry testing (+84.0° to +87.5°) is conducted as a critical indicator of identity and quality control.
● Moisture and Residue Testing
Water content (≤2.0%) and ash content (≤0.7%) are determined to evaluate excipient purity and long-term stability.
● Physicochemical Properties and Stability
Solubility, melting point, TGA, and DSC provide detailed profiles of thermal behavior and physicochemical stability. Additional tests evaluate performance under heat, acidic environments, and formulation conditions.
● Application Performance Evaluation
For pharmaceuticals, we test formulation stability and excipient compatibility. For food and beverage products, we assess sweetness retention and functionality in diverse formulations.
Why Choose MtoZ Biolabs?
✅ Comprehensive Platforms: HPLC, LC-MS/MS, GC-MS, FTIR, NMR, TGA, DSC.
✅ Tailored Testing Plans: Flexible combinations to meet pharmaceutical and food industry needs.
✅ Integrated Interpretation: Quantitative results combined with functional analysis and formulation-specific recommendations.
✅ One-Time-Charge: Our pricing is transparent, no hidden fees or additional costs.
Sample Submission Suggestions
For Sucralose Analysis Service, MtoZ Biolabs accepts powders, crystalline materials, pharmaceutical formulations (including tablets, oral liquids, and coatings), as well as food and beverage samples. To ensure accuracy, all samples should be sealed, moisture-free, and kept away from strong acids or bases. Powders are generally suitable for shipping at ambient temperature, while liquid samples may require either ambient or refrigerated transport depending on their stability.
Contact MtoZ Biolabs to receive detailed submission guidelines and ensure your samples are prepared correctly for reliable testing.
Applications
· Pharmaceutical Development and QC: Verify batch consistency and regulatory compliance for excipient use.
· Food and Beverage R&D: Evaluate sweetness performance and stability in different formulations.
· Nutrition and Health: Support safety assessments for low-calorie and diabetic-friendly products.
· Process and Formulation Optimization: Monitor content and stability to guide production improvements.
MtoZ Biolabs Sucralose Analysis Service provides comprehensive and actionable data for both pharmaceutical and food applications. By combining advanced instrumentation with expert interpretation, we help clients ensure excipient quality, support formulation development, and achieve regulatory compliance.
Beyond sucralose, our analytical capabilities also cover hydroxypropyl methylcellulose, ethylcellulose, microcrystalline cellulose, and other excipients, enabling complete solutions for formulation optimization, quality control, and industrial translation.
FAQ
Q1: What are the key quality control indicators for sucralose?
Critical indicators include purity, specific rotation, moisture content, and impurity profile, as these directly affect its safety and stability when used as a pharmaceutical excipient or food additive.
Related Services
How to order?







