SDS-PAGE Peptide Purity Analysis Service
- Peptide Drug Development
- Vaccine and Immunopeptide Research
- Protein Degradation Analysis
- Natural Bioactive Peptide Screening
- Process Optimization
SDS-PAGE Peptide Purity Analysis Service refers to a technical service that utilizes SDS-PAGE technology to perform molecular weight-based separation and visual band analysis of peptide samples, thereby assessing their purity, degradation status, and impurity composition.
Peptides play an increasingly important role in drug development, biomaterials, immunotherapy, and nutritional sciences. Due to their structural complexity, functional diversity, and low molecular weight, accurate evaluation of peptide purity and structural integrity has become a critical step in product development and quality control. During peptide synthesis, expression, isolation, and preparation, incomplete synthesis byproducts, degradation fragments, polymers, or other impurities frequently arise. These impurities may significantly affect biological activity, safety, or the outcomes of downstream experiments.
Among the various purity analysis methods, SDS-PAGE (Sodium Dodecyl Sulfate-Polyacrylamide Gel Electrophoresis) is a classical technique for protein and peptide separation. It offers advantages such as methodological maturity, intuitive results, ease of operation, and high reproducibility. By incorporating the Tricine-SDS-PAGE electrophoresis system, this technique has been optimized for analyzing peptides within the 1–30 kDa molecular weight range, making it particularly suitable for the separation and purity assessment of low-molecular-weight peptides. As a result, it is widely applied in the quality evaluation and process optimization of peptide raw materials and finished products.
MtoZ Biolabs provides SDS-PAGE Peptide Purity Analysis Service to support purity assessment and quality control of peptide samples. This service is applicable to synthetic peptides, natural peptides, and recombinant peptides across a broad molecular weight range. Through electrophoretic separation and image analysis, it accurately reveals the distribution of major components and impurity peptides, offering efficient and reliable technical support for peptide research, process development, and stability verification.
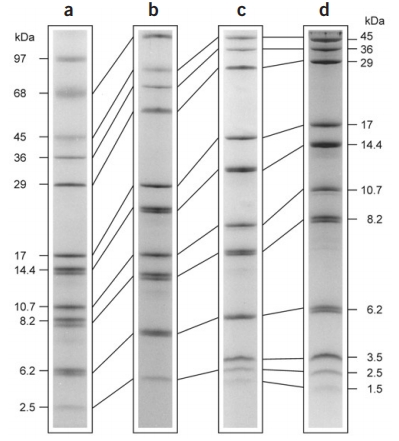
Schägger H. Nat Protoc. 2006.
Figure 1. Tricine-SDS-PAGE of marker proteins using various types of gel.
Analysis Workflow
The general analysis workflow of SDS-PAGE Peptide Purity Analysis Service is as follows:
1. Sample Preprocessing
Mix the peptide sample with SDS buffer and reducing agents to denature the peptides.
2. Gel Selection and Preparation
Choose the appropriate acrylamide concentration (typically 10%–16.5%) based on the target peptide's molecular weight and prepare the Tricine electrophoresis buffer system. The sample is then loaded into the gel wells.
3. Electrophoresis Run
Perform electrophoresis under constant voltage conditions to ensure complete separation of the sample.
4. Staining and Development
Use Coomassie Brilliant Blue or silver staining methods to enhance sensitivity and clearly visualize the bands.
5. Image Capture and Result Analysis
Capture gel images and analyze the number, intensity, and distribution of bands to assess sample purity and identify potential impurities or degradation products.
Service Advantages
High-resolution separation of small peptides: Using the Tricine buffer system, it effectively separates low molecular weight peptides with a resolution superior to traditional SDS-PAGE.
Flexible staining options: Depending on sample abundance, methods such as Coomassie Brilliant Blue or silver staining can be selected, balancing detection sensitivity with cost-effectiveness.
Low sample requirement and strong adaptability: Only microgram-level samples are needed, suitable for synthetic peptides, natural peptides, recombinant expressed peptides, and their formulations.
Compatible with other services: Supports integration with subsequent techniques like mass spectrometry, providing a one-stop solution from purity assessment to structural analysis.
Sample Submission Suggestions
Sample Types: Supports synthetic peptides, naturally sourced peptides, recombinant expressed peptides, etc.
Storage and Transportation: Store at low temperatures (−20°C or −80°C). Dry ice transportation is recommended to avoid repeated freeze-thaw cycles.
Additional Notes: We recommend contacting us prior to sample submission for detailed and tailored sample preparation guidelines.
Applications
Application examples of SDS-PAGE Peptide Purity Analysis Service:
Confirm the purity and stability of synthetic peptides to meet quality control requirements.
Analyze the purity of antigen peptides or epitope peptides' expression products, supporting quality control of vaccine components and evaluating immune response reliability.
Monitor the peptide composition and distribution in proteolytic products, providing foundational data for subsequent analysis.
Perform preliminary purity assessment of crude peptide extracts from animal, plant, or microbial sources.
Compare the impact of different purification processes on product purity.
Deliverables
1. Comprehensive Experimental Details
2. Materials, Instruments, and Methods
3. Total Ion Chromatogram & Quality Control Assessment (project-dependent)
4. Data Analysis, Preprocessing, and Estimation (project-dependent)
5. Raw Data Files
* For research use only. Not for diagnostic purposes. Samples from individual customers or for personal use are not accepted.
Related Services
How to order?







