Resources
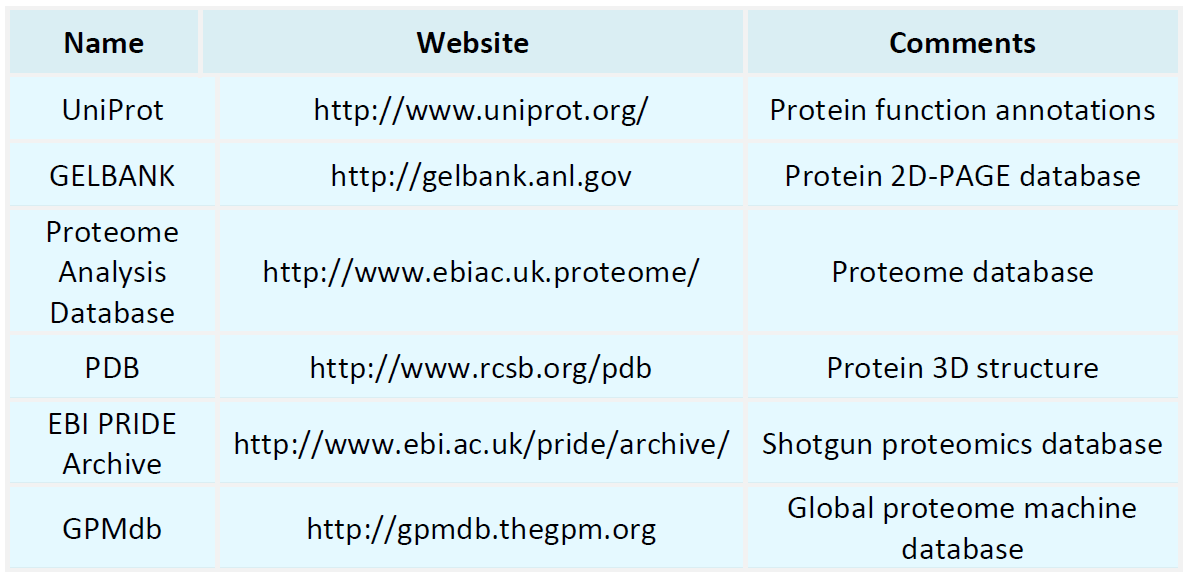
Proteomics Databases
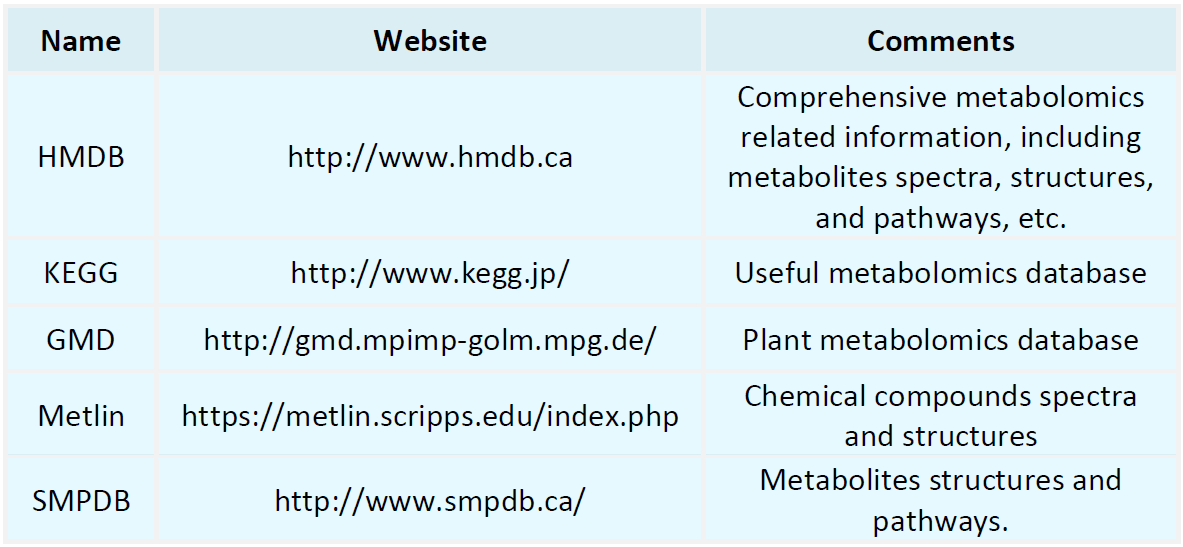
Metabolomics Databases
-
• Detection of Protein Modifications by Top-Down Proteomics
Protein modification is a vital regulatory mechanism of protein function and activity within organisms. Post-translational modifications (PTMs), such as phosphorylation, acetylation, methylation, and ubiquitination, allow precise control over protein functions. These modifications not only play crucial roles in cellular signaling, metabolic regulation, and disease development, but also open new avenues for drug discovery and biomarker identification.
-
• Quantitative Analysis of Protein Oxidative Modifications Using Mass Spectrometry
Protein oxidative modifications are a common form of post-translational modification, playing crucial roles in regulating protein function, cellular signaling, and the pathogenesis of various diseases. Therefore, understanding and quantifying protein oxidative modifications are key to elucidating the mechanisms of redox balance in cells and their implications for disease.
-
• MS Analysis of Unknown Protein Identification
Mass spectrometry (MS) serves as a highly sensitive and high-resolution analytical technique, playing a crucial role in the identification of unknown proteins.
-
• Analytical Techniques for Unknown Protein Identification
The identification of unknown proteins is crucial in modern biological research. With advancements in genomics and proteomics, a vast amount of protein sequence data is available, yet understanding these proteins' functions remains challenging. This paper explores major analytical techniques used in protein identification, including mass spectrometry, X-ray crystallography, nuclear magnetic resonance (NMR) spectroscopy, and bioinformatics methods, highlighting their advantages and limitations.
-
• Workflow of Quantitative Tyrosine Phosphoproteomics Analysis
Protein phosphorylation is a critical modification in cellular signaling, metabolic regulation, and other biological processes. Tyrosine phosphorylation, in particular, plays a significant role in various biological contexts, including cell growth, differentiation, and cancer. Quantitative tyrosine phosphorylation proteomics is a powerful technique for comprehensively studying the levels and dynamics of tyrosine phosphorylation.
-
• 4D DIA Proteomics: Comprehensive Overview of Protein Quantification and Differential Analysis
Introduction Proteomics is the scientific field that studies the composition, structure, and function of all proteins in an organism. With the continuous development of technology, quantitative and differential analysis of proteomics has become an important research direction in the field of biopharmaceuticals.
-
• 4D-DIA Label-Free Quantitative Proteomics: the Key Technology in Proteomics
Proteomics is the discipline that studies the composition, structure, function, and interactions of all proteins in an organism. In recent years, with advancements in technology and continuous innovation in methods, the field of proteomics has made significant progress. Among them, 4D-DIA non-targeted quantitative proteomics, as a key technology, has been widely applied in the field of biopharmaceuticals.
-
• Best Practice Sharing: How to Conduct Anti-Drug Antibody Testing
Anti-Drug Antibody (Ada) refers to antibodies produced by the human immune system in response to exogenous drugs, such as biopharmaceuticals. These antibodies may impact the efficacy and safety of the drugs, making it crucial to detect and monitor the presence of Anti-Drug Antibodies (Ada).
-
• Unveiling Circular Dichroism Data Processing: Analysis of Techniques and Diversity
Circular dichroism spectroscopy is an important analytical technique widely used in the field of biopharmaceuticals. It provides valuable information about molecular structure, conformation, and interactions. However, processing and analysis of circular dichroism spectroscopy data is not an easy task.
-
• Applications of Circular Dichroism: Peptide Mapping Workflow Reveals Diversity
Introduction Peptide mapping analysis is an important technique in the field of biopharmaceutical research, helping scientists to gain a deeper understanding of the structure and function of proteins and their constituent amino acid sequences. Circular dichroism spectroscopy, as a powerful analytical tool, can provide valuable information about the conformation of biomolecules.
How to order?







