N/C Terminal Sequencing Service
N- and C-terminal sequences are important structures and functional parts of protein and polypeptide, and they may play decisive roles in the biological function of protein. The main methods for protein C-terminal sequencing include carboxypeptidase, chemical and tandem mass spectrometry, and the common methods for N-terminal sequencing include Edman degradation sequencing and mass spectrometry. Each of these methods has the respective advantage and shortcoming. Therefore, the combination of a variety of different sequencing methods can adapt to the requirements of multiple protein sequencing. For example, Edman degradation may not well solve the problem of blockage and protein modification on the N-terminal end. This drawback can be overcomed by mass spectrometry analysis. MtoZ Biolabs uses both Edman degradation system and MALDI-TOF for N/C sequencing of biopharmaceutical products. This combination of two methods ensures accurate analysis of N-terminal blockage and modified protein N/C terminal.
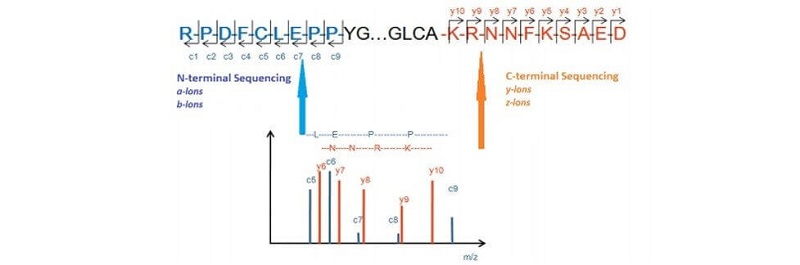
Figure 1. Biopharmaceutical N/C-Terminal Sequencing
Sample Submission Requirements
1. Sample Types: Lyophilized & Solution Samples
2. Amount of Total Protein: >300 ug
3. Purity: >90%
4. Content of Salt: Volatile Salt<200 mM; Non-Volatile Salt<5 mM
Case Study
In this case, the N- and C-terminal sequence of one of our clients' protein sample has been analyzed using a combination of Edman degradation and MALDI-TOF technologies. The sequencing data highlighted in red color accurately shows the N- and C-terminal sequence of the protein.

Deliverables
1. Experiment Procedures
2. Parameters of Liquid Chromatography and Mass Spectrometer
3. Edman Sequencing and MS Raw Data Files
4. Biopharmaceutical N/C-Terminal Sequence Results
5. Bioinformatics Analysis
Related Services
Identification of Biopharm
Variation Analysis
Purity Analysis
How to order?







