Peptide N-terminal Sequencing Service
- Edman Degradation: Sequentially cleave N-terminal residues and identify each PTH-amino acid via chromatography.
- Mass Spectrometry: Digest peptides, acquire MS/MS spectra, and perform sequence reconstruction and database matching.
- Synthetic Peptide Quality Control: Verify the accuracy of the N-terminal sequence to eliminate synthesis errors.
- N-terminal Modification Studies: Qualitative and quantitative analysis of post-translational modifications such as acetylation and formylation.
- Biomarker Development: Precisely define peptide sequences to support the identification of disease biomarkers.
- Antibody or Vaccine Component Verification: Confirm structural consistency of epitope peptides used as antigens.
Peptide N-terminal Sequencing Service is a technical service that focuses on analyzing the N-terminal amino acid sequence of a peptide chain, aiming to confirm the sequence integrity and start site accuracy of synthetic peptides or proteins. This service has important application value in the fields of peptide drug development, protein structure and function research, post-translational modification verification, and antibody development.
In peptide research, the N-terminal sequence not only determines the molecule’s spatial conformation and functional activity but also affects its biological stability, degradation pathway, and recognition capability. Especially in applications involving synthetic peptide production, fusion protein expression, or vaccine antigen development, confirming proper N-terminal exposure or the presence of modifications (e.g., acetylation, formylation) is critical for subsequent functional analysis.

Landreh M. et al. Biomolecular Concepts. 2014.
Figure 1. The three-dimensional structure of C-peptide.
N-terminal sequencing primarily relies on two complementary techniques, each suited for different sample types and research objectives:
1. Edman Degradation
This is a classic stepwise chemical degradation method that uses phenyl isothiocyanate (PITC) to specifically label the free N-terminal amino group. Under basic conditions, one amino acid is cleaved per cycle and identified via chromatography. It is suitable for unmodified short peptides (typically 30–50 amino acids).
2. Mass Spectrometry
Platforms such as LC-MS/MS or MALDI-TOF/TOF are used to deduce the N-terminal sequence by interpreting peptide fragment ion spectra. This method is particularly effective for detecting N-terminal modifications, blockages, or incomplete sequences, and offers high throughput and sensitivity.
Leveraging Edman degradation and high-resolution mass spectrometry platforms, MtoZ Biolabs offers Peptide N-terminal Sequencing Service to deliver high-accuracy N-terminal sequence determination for various peptide types. The service accommodates diverse sample sources, modification states, and research goals, fulfilling the needs of both academic and industrial users for high-quality sequence information.
Analysis Workflow
The main workflow of Peptide N-terminal Sequencing Service is as follows:
1. Sample Preparation and Purification
Remove salts, surfactants, and other interfering substances, and improve sample purity using methods such as SEC or HPLC.
2. Method Selection and Preprocessing
Choose between Edman degradation or mass spectrometry based on sample characteristics and research objectives, followed by appropriate sample preprocessing.
3. Sequence Determination
4. Data Validation and Reporting
Interpret the preliminary sequence using specialized software, verify accuracy with background information, and generate a comprehensive result report.
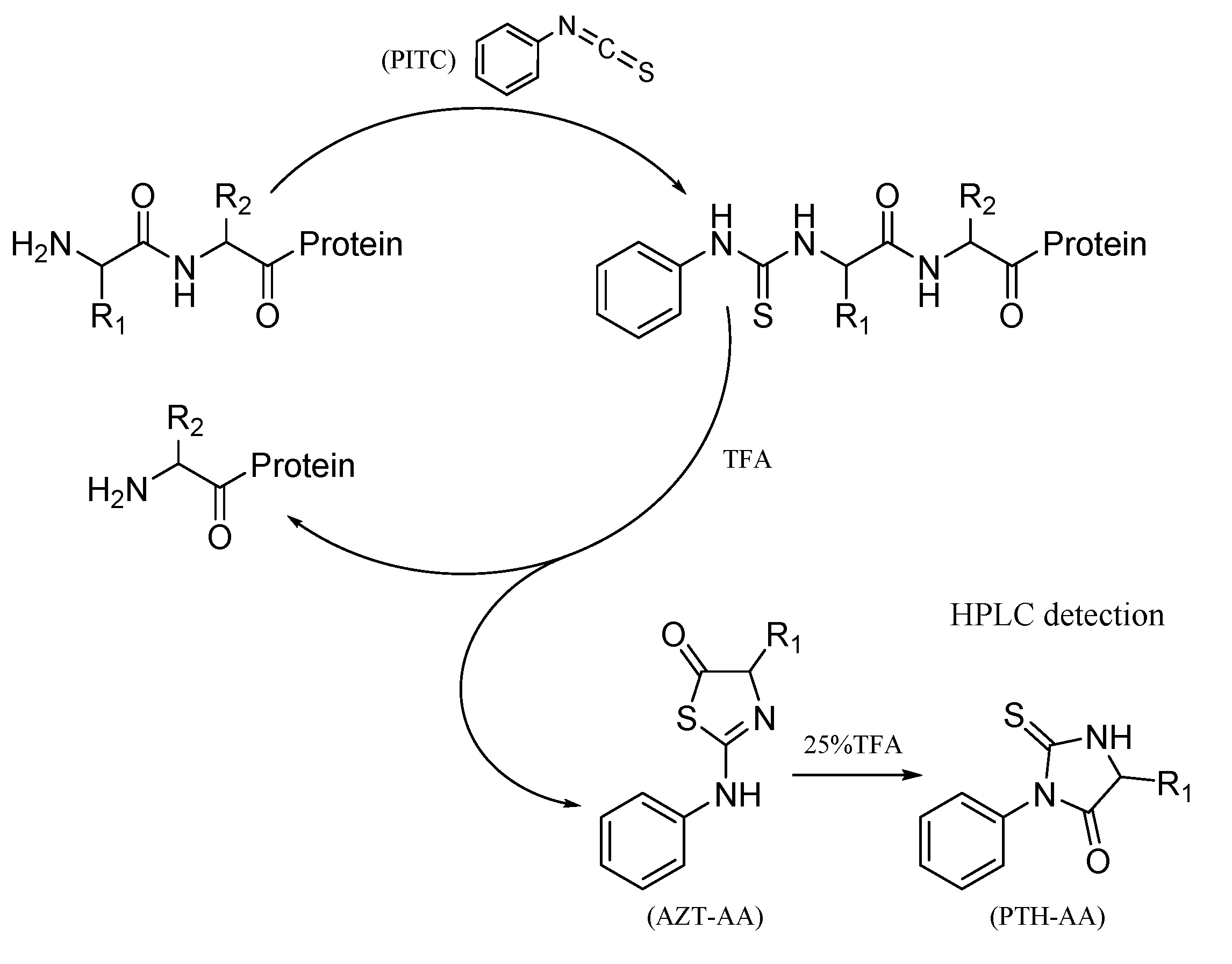
Yang W. et al. Molecules. 2021.
Figure 2. N-terminal sequencing cycle in the Edman degradation assay.
Service Advantages
Advanced Analysis Platform: MtoZ Biolabs established an advanced Peptide N-terminal Sequencing Service platform, guaranteeing reliable, fast, and highly accurate analysis service.
One-Time-Charge: Our pricing is transparent, no hidden fees or additional costs.
High-Data-Quality: Deep data coverage with strict data quality control. AI-powered bioinformatics platform integrates all Peptide N-terminal Sequencing data, providing clients with a comprehensive data report.
Capable of Identifying N-terminal Modifications and Truncations: Effectively detects N-terminal post-translational modifications (e.g., acetylation, formylation) and unintended N-terminal truncations.
Complementary Multi-Platform Validation System: Offers a dual sequencing strategy using both Edman degradation and mass spectrometry, allowing cross-validation or flexible switching based on sample limitations to enhance sequencing efficiency and data reliability.
Sample Submission Suggestions
Sample Types: Supports synthetic peptides and peptides derived from natural sources.
Storage and Shipping: Store samples at low temperature (−80 °C) in either lyophilized or solution form. Shipping on dry ice is recommended. Avoid repeated freeze–thaw cycles.
Additional Notes: We recommend contacting us prior to sample submission for detailed and tailored sample preparation guidelines.
Applications
Peptide N-terminal Sequencing Service has a wide range of applications, including:
Deliverables
1. Comprehensive Experimental Details
2. Materials, Instruments, and Methods
3. Total Ion Chromatogram & Quality Control Assessment (project-dependent)
4. Data Analysis, Preprocessing, and Estimation (project-dependent)
5. Bioinformatics Analysis
6. Raw Data Files
Related Services
How to order?







