Peptide Drug In Vivo Toxicity Assessment Service
MtoZ Biolabs provides Peptide Drug In Vivo Toxicity Assessment Service to support early safety evaluation of peptide drug candidates under physiologically relevant conditions. Our studies characterize dose-related toxicity, systemic responses, and biological tolerability, enabling informed decisions during discovery and preclinical development. With flexible study designs and controlled analytical workflows, we generate high-quality data that guide candidate selection and reduce late-stage risks.
Overview
In vivo toxicity assessment is essential for identifying safety liabilities that cannot be predicted through in vitro screening alone. Peptide drugs may produce unexpected systemic responses, including organ-specific effects, metabolic imbalance, or immune-related reactions. Early in vivo evaluation helps establish safe exposure limits, identify early warning signals, and refine dose ranges for further R&D activities.
Animal studies routinely incorporate clinical observations, behavior monitoring, body weight trends, hematology, clinical chemistry, and histopathological evaluation. These measurements provide an integrated view of peptide tolerability and help reduce attrition as candidates advance through development. MtoZ Biolabs applies well-established in vivo models with the flexibility to accommodate both standard screening needs and customized toxicology designs.
Peptide Drug In Vivo Toxicity Assessment Service at MtoZ Biolabs
MtoZ Biolabs offers a structured portfolio of in vivo toxicity assessments tailored to peptide drug characteristics and project requirements.
1. Single Dose Toxicity Assessment (Acute Toxicity)
Short-term evaluation to identify immediate toxic effects and approximate safety margins.
2. Repeated Dose Toxicity Assessment
Multi-day or multi-week dosing studies designed to identify cumulative or delayed toxicity.
3. Sub-chronic and Chronic Toxicity Evaluation
Extended studies assessing long-term tolerability, functional impairment, or organ-level stress.
4. Specialized Toxicity Profiling
Optional evaluation of nephrotoxicity, neurotoxicity, or mitochondrial toxicity based on study needs.
MtoZ Biolabs can also support customized in vivo study designs for peptide candidates requiring unique dosing regimens or specialized monitoring parameters.
Why Choose MtoZ Biolabs?
✅ Peptide Toxicity Experience: Practical experience supporting toxicity studies across multiple peptide formats and dosing approaches.
✅ Integrated Assessment Workflow: Clinical observations, pathology testing, and exposure measurements coordinated within a unified study framework.
✅ Appropriate Animal Models: Validated models suitable for multiple administration routes commonly used for peptide therapeutics.
✅ Clear and Structured Data Output: Data organized to support dose comparisons and early development decision-making.
✅ Steady Project Coordination: Consistent communication, organized scheduling, and dependable operational support.
✅ One-Time-Charge: Our pricing is transparent, no hidden fees or additional costs.
Start Your Project with MtoZ Biolabs
MtoZ Biolabs delivers high-quality Peptide Drug In Vivo Toxicity Assessment Service results that support dose justification, safety profiling, and preparation for advanced preclinical studies.
Contact us to initiate your toxicity assessment project.
FAQ
Q1: What types of samples are suitable?
Purified peptide drugs, lyophilized peptides, or well-characterized peptide formulations prepared in suitable vehicles. A minimum of 1 mg of solid peptide or 1 mL of solution is recommended.
Q2: What is the service general workflow?
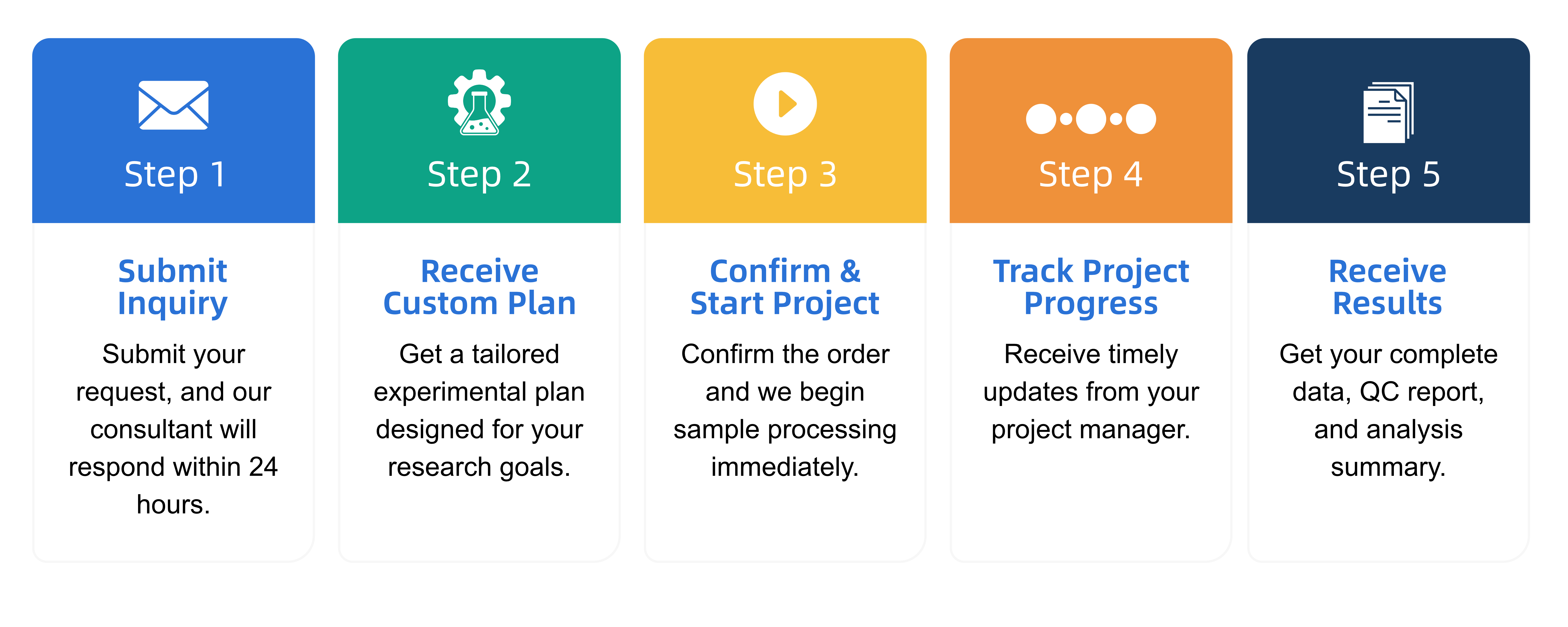
Q3: What data formats are provided?
Deliverables include raw animal-level data, summarized toxicity tables, clinical pathology results, histopathology findings, dose analysis outputs, and full study reports in Excel, CSV, and PDF formats.
Q4: How should I prepare my samples?
· Provide materials with documented purity and identity
· Include formulation details, solubility information, and storage conditions
· Supply sufficient quantity for the full dosing schedule
· Ship materials under appropriate temperature-controlled conditions
· Notify us of any specific formulation or handling requirements
For more information, please refer to Sample Submission Guidelines for Proteomics. If you are unsure about sample preparation, our team is available for consultation and can guide you through the process to ensure optimal results.
Related Services
How to order?







