Peptide C-terminal Sequencing Service
- Peptide Drug Development and Validation: Confirm the C-terminal structure of candidate peptide drugs to verify synthesis completeness and modification status, ensuring accurate and effective bioactive peptide design.
- Protein Hydrolysate Studies: Identify specific C-terminal peptides generated by proteolytic cleavage to support investigations of protein degradation pathways and cleavage site mapping.
- Post-Translational Modification (PTM) Analysis: Detect C-terminal modifications such as amidation or carboxymethylation to understand their roles in peptide stability, subcellular localization, and functional regulation.
- Antibody or Vaccine Peptide Screening: Confirm the structure of C-terminal epitope peptides to support the design and quality control of peptide-based vaccines or immunoassays.
- Discovery and Functional Characterization of Natural Bioactive Peptides: Determine the full sequence of novel peptides extracted from animals, plants, or microorganisms by resolving their C-termini, laying the groundwork for structure–function relationship studies.
Peptide C-terminal Sequencing Service is a specialized service that utilizes advanced technologies such as mass spectrometry to analyze the sequence of peptides at the carboxyl terminus (C-terminus). This service is widely applied in functional peptide research, peptide drug development, synthetic peptide quality control, and structural verification of protein cleavage products generated in vitro.
In areas such as peptide drug discovery, functional peptide screening, and synthetic peptide quality assessment, C-terminal sequence information is critical for evaluating peptide integrity, biological activity, and stability. Unlike conventional N-terminal sequencing, C-terminal sequencing presents greater technical challenges due to the lack of universal and efficient sequential enzymatic cleavage strategies. It typically relies on high-resolution mass spectrometry (e.g., LC-MS/MS) combined with techniques such as targeted enzymatic digestion, derivatization labeling, or chemical modification to infer and validate peptide sequences.

Landreh M. et al. Biomolecular Concepts. 2014.
Figure 1. The three-dimensional structure of C-peptide.
Leveraging advanced chromatography and mass spectrometry platforms, MtoZ Biolabs offers Peptide C-terminal Sequencing Service to enable sensitive analysis of C-terminal peptide sequences. This service provides accurate and reliable structural information on the peptide C-terminus, supporting in-depth investigations in structure confirmation, modification identification, and mechanistic studies.
Analysis Workflow
The main workflow of Peptide C-terminal Sequencing Service is as follows:
1. Sample Preparation
Perform buffer exchange and concentration to remove impurities and prepare the sample for enzymatic digestion and mass spectrometry analysis.
2. Targeted Enzymatic Digestion
Select specific enzymes for directional cleavage to preserve or expose the C-terminal sequence.
3. LC-MS/MS Analysis
Separate and detect peptide fragments using high-resolution liquid chromatography–tandem mass spectrometry, and collect MS/MS fragmentation data.
4. Data Analysis
Identify peptide sequences through database searching and manual verification to accurately determine the C-terminal sequence of the target peptide.
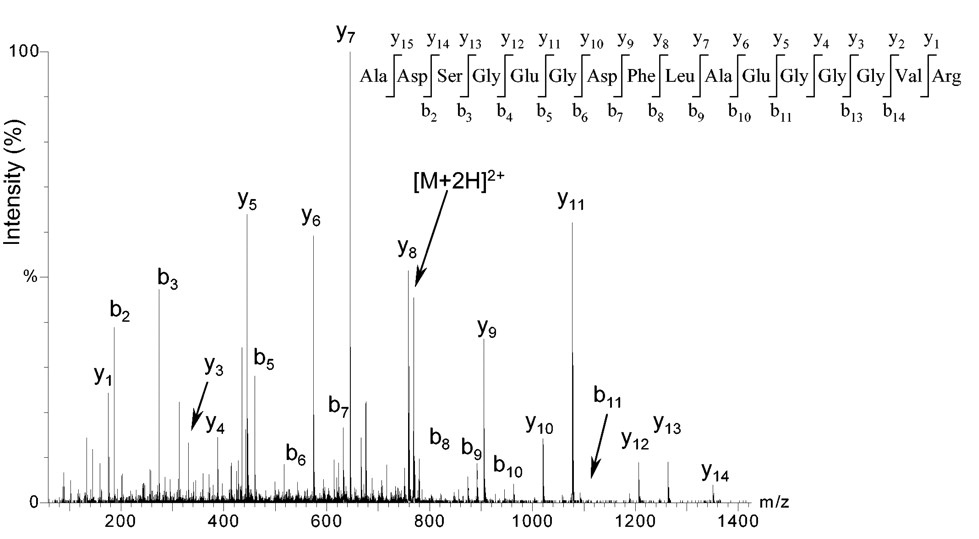
Cristea IM. et al. Blood. 2004.
Figure 2. Sequencing of peptide using MS/MS.
Service Advantages
Advanced Analysis Platform: MtoZ Biolabs established an advanced Peptide C-terminal Sequencing Service platform, guaranteeing reliable, fast, and highly accurate analysis service.
One-Time-Charge: Our pricing is transparent, no hidden fees or additional costs.
High-Data-Quality: Deep data coverage with strict data quality control. AI-powered bioinformatics platform integrates all Peptide C-terminal Sequencing data, providing clients with a comprehensive data report.
Multi-Strategy Integration: Depending on the sample characteristics, the service flexibly incorporates approaches such as targeted enzymatic digestion, C-terminal labeling, and chemical derivatization to enhance sequencing specificity and success rate.
Fully Customizable Workflow: Every step of the service can be tailored to project needs, including enzyme selection, analysis depth, and reporting format, ensuring precise alignment with research objectives.
Sample Submission Suggestions
Sample Types: Supports synthetic peptides and peptides derived from natural sources.
Storage and Shipping: Store samples at low temperature (−80 °C) in either lyophilized or solution form. Shipping on dry ice is recommended. Avoid repeated freeze–thaw cycles.
Additional Notes: We recommend contacting us prior to sample submission for detailed and tailored sample preparation guidelines.
Applications
Application examples of Peptide C-terminal Sequencing Service:
Deliverables
1. Comprehensive Experimental Details
2. Materials, Instruments, and Methods
3. Total Ion Chromatogram & Quality Control Assessment (project-dependent)
4. Data Analysis, Preprocessing, and Estimation (project-dependent)
5. Bioinformatics Analysis
6. Raw Data Files
Related Services
How to order?







