Parallel Reaction Monitoring (PRM): Principles, Workflow, and Applications
Parallel reaction monitoring (PRM) is a targeted quantitative proteomics technique based on high-resolution mass spectrometry. In recent years, it has gained widespread application in functional protein studies, biomarker validation, and targeted quantification within complex biological matrices. Compared to multiple reaction monitoring (MRM), PRM offers significant advantages in specificity, resolution, and interference resistance, making it particularly suited for high-precision quantification of target peptides.
This article provides a comprehensive overview of PRM from four perspectives: its fundamental principles, experimental workflow, technical advantages, and representative applications. We also highlight the unique features of MtoZ Biolabs’ PRM-based proteomics services.
Principles of PRM: A Multi-Dimensional “Snapshot” of Target Peptides
1. Core Technology: Comprehensive Fragment Ion Acquisition + High-Resolution Accurate Mass Detection
PRM employs high-resolution and high-accuracy mass spectrometers such as Orbitrap or Q-TOF to selectively fragment precursor ions of target peptides, capturing all resulting fragment ion signals in parallel. Unlike MRM, which depends on predefined ion transitions, PRM enables the acquisition of complete fragment ion spectra in a single run, significantly enhancing data specificity and reliability.
This comprehensive acquisition approach provides PRM with superior anti-interference capabilities, particularly in complex biological samples where ion interference is unpredictable.
2. PRM vs. MRM: A Comparative Summary
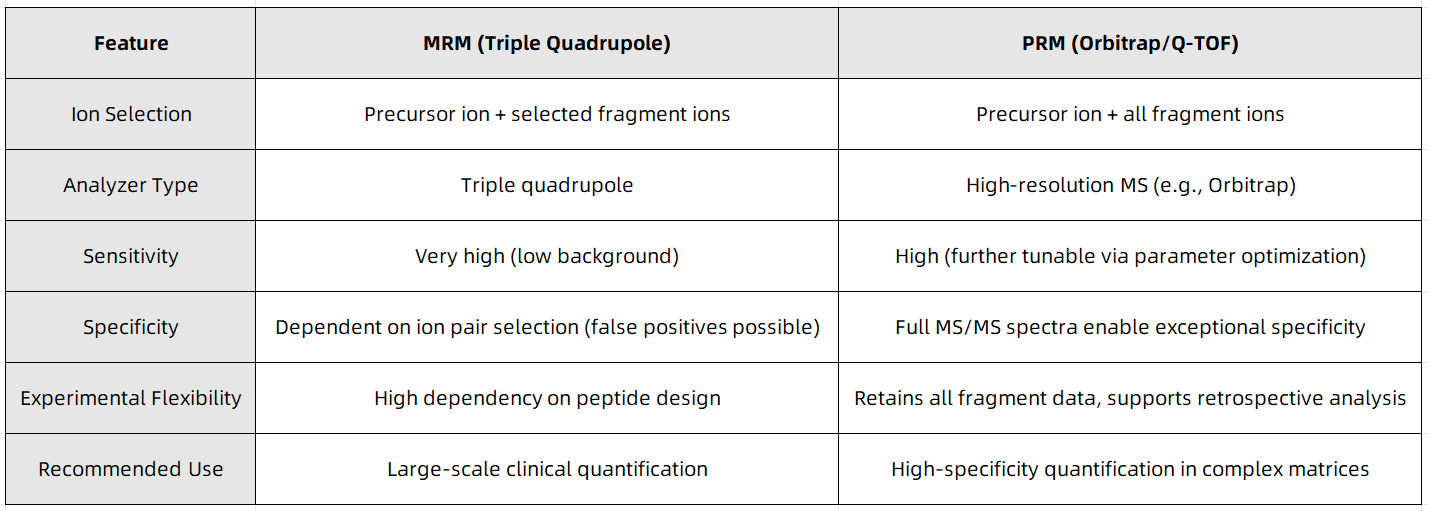
Figure 1
PRM Workflow: From Target Design to Quantitative Analysis
1. Target Selection and Peptide Design
(1) Source: DDA/DIA discovery phases, literature, or peptide databases (e.g., PeptideAtlas, SRMAtlas)
(2) Selection criteria:
①Uniqueness (proteotypic peptides)
②Good ionization efficiency
③Peptides that generate stable fragment ions upon cleavage
2. Standard Selection (Optional)
(1) Incorporation of stable isotope-labeled peptides (SIS) for absolute quantification
(2) Internal standard peptides for correction of sample processing or MS drift
3. Sample Preparation
Typically includes: Protein extraction → Tryptic digestion → Peptide purification → Internal standard spiking → MS analysis
4. Mass Spectrometry Analysis (Orbitrap PRM)
(1) High-resolution MS (e.g., Thermo Orbitrap Exploris)
(2) Set precursor m/z values of target peptides; collect full fragment ion spectra automatically
(3) Output: High-resolution, highly specific chromatographic peak maps
5. Data Processing
(1) Main software: Skyline
(2) Validation and quantification based on retention time, peak shape, and consistency across multiple fragment ions
(3) Delivers relative or absolute quantification results
Technical Advantages of PRM
1. High Specificity
The use of full-spectrum MS/MS and high resolution significantly reduces false positives. PRM is well-suited for detecting low-abundance proteins or quantifying targets within complex biological matrices (e.g., plasma, urine).
2. Exceptional Flexibility
Only precursor ions need to be specified; all fragment ions are retained, allowing for retrospective data mining and method optimization.
3. No Need for Transition List Development
PRM eliminates the time-consuming ion pair optimization required in MRM, shortening the method development cycle.
4. Complementary to DIA
PRM is ideal for validation, whereas DIA excels in discovery. The combination enables a highly efficient discovery-to-validation pipeline.
Representative Applications of PRM
1. Biomarker Validation
Frequently used to verify targets identified through DDA or DIA, especially for secondary quantification of differentially expressed proteins.
2. Targeted Quantification in Complex Samples
PRM provides high specificity for quantifying targets in complex biological fluids such as plasma, urine, and cerebrospinal fluid.
3. Pharmacodynamics and Time-Course Studies
Enables dynamic profiling of pathway-related proteins before and after drug treatment.
4. Quantification in Synthetic Biology
Allows accurate measurement of key enzymes in metabolic pathways to inform regulatory strategy design.
MtoZ Biolabs: PRM Proteomics Service Highlights
1. High-Resolution PRM Platform
Equipped with Thermo Orbitrap Exploris 480.
2. Standardized Workflow
End-to-end standardization from sample processing to peptide selection, internal standard incorporation, and data analysis
3. Supports Absolute Quantification
Offers synthesis of stable isotope-labeled peptides for clinical validation
4. Integrates with DIA Data
Enabling seamless transition from target discovery to verification and translational application
Conclusion: Enhancing Quantitative Proteomics with PRM
Parallel reaction monitoring (PRM) is a powerful targeted quantification strategy that integrates the strengths of high-resolution mass spectrometry. It combines high sensitivity, specificity, and stability, making it particularly well-suited for biomarker validation and low-abundance protein quantification. As its utility in clinical research and complex sample analysis continues to grow, PRM is becoming an indispensable tool in modern proteomics. Through strategic target design, optimized workflows, and advanced platforms, researchers can achieve more stable and accurate protein quantification. MtoZ Biolabs is committed to delivering reliable, high-quality PRM solutions that drive precision research and translational advancement.
MtoZ Biolabs, an integrated chromatography and mass spectrometry (MS) services provider.
Related Services
How to order?







