Mass Spectrometry Quantification Service
- Pharmaceutical Development and Quality Control: Ensuring drug purity, impurity detection, and metabolite profiling to ensure drug quality.
- Environmental Monitoring: Efficiently detecting harmful substances in water, air, and soil, supporting environmental protection efforts.
- Food Safety Testing: Accurately identifying contaminants, additives, and allergens in food products, ensuring consumer safety.
- Clinical and Life Sciences Research: Used for biomarker detection and drug concentration monitoring, advancing precision medicine.
- Cosmetics Industry: Ensuring cosmetic ingredients meet safety standards, improving product compliance.
Mass spectrometry quantification is a quantitative analysis technique based on mass spectrometry, widely applied in fields such as biopharmaceuticals, chemical analysis, and environmental monitoring. By converting the molecules in a sample into ions and analyzing them based on their mass-to-charge ratio (m/z), mass spectrometry quantification can accurately determine the concentration or content of target substances in the sample, making it particularly suitable for trace analysis in complex samples.
In impurity analysis, mass spectrometry quantification offers unmatched advantages. It effectively identifies and quantifies impurities in samples, whether they are small or large molecules. This technology provides high sensitivity and accuracy in quantification while ensuring high reproducibility and reliability throughout the analysis process. Whether for raw material validation in drug development or contaminant detection in environmental monitoring, mass spectrometry quantification is an essential tool.
As a specialized biotechnology service provider, MtoZ Biolabs is committed to delivering precise Mass Spectrometry Quantification Service for research and industrial clients. Utilizing advanced mass spectrometry platforms (such as LC-MS/MS, Orbitrap MS, ICP-MS) and a rigorous quality control system, we ensure that every analysis meets industry standards, helping clients overcome challenges in impurity analysis, component quantification, and more.
Services at MtoZ Biolabs
At MtoZ Biolabs, we provide comprehensive Mass Spectrometry Quantification Service covering the entire workflow from sample preparation and instrument analysis to data interpretation and report generation. Our services include:
· Impurity Quantification: Quantifying impurities in products such as pharmaceuticals, chemicals, and food, assisting with quality control and compliance testing.
· Component Quantification: Precisely measuring the content of individual components within a sample, based on client specifications, providing accurate data for quality analysis.
· Drug Metabolite Analysis: Ideal for biopharmaceutical development, assisting with the identification and quantification of major drug metabolites.
· Complex Matrix Analysis: Handling challenging samples like serum and tissue to provide accurate quantitative data, supporting preclinical and clinical studies.

Why Choose MtoZ Biolabs?
1. Multi-Platform Technology
We utilize various mass spectrometry techniques, including LC-MS/MS, Orbitrap MS, and ICP-MS, to provide precise impurity quantification for diverse sample types.
2. High Sensitivity and Precision
Our mass spectrometers detect impurities at ng/mL levels, ensuring accurate quantification of trace compounds.
3. High-Data-Quality
Deep data coverage with strict data quality control. AI-powered bioinformatics platform integrate all mass spectrometry quantification data, providing clients with a comprehensive data report.
4. Customized Service
Tailored to the specific research needs of our clients, we offer flexible experimental design and personalized data analysis to ensure the achievement of research goals to the fullest extent.
5. One-Time-Charge
Our pricing is transparent, no hidden fees or additional costs.
Applications
Mass Spectrometry Quantification Service provide accurate and reliable analysis across various industries, ensuring precise measurements for a wide range of applications.
Case Study
Case 1: Quantification of 7-nitro impurity in sitagliptin via UPLC-MS/MS, providing a precise solution for drug impurity detection and quality control.
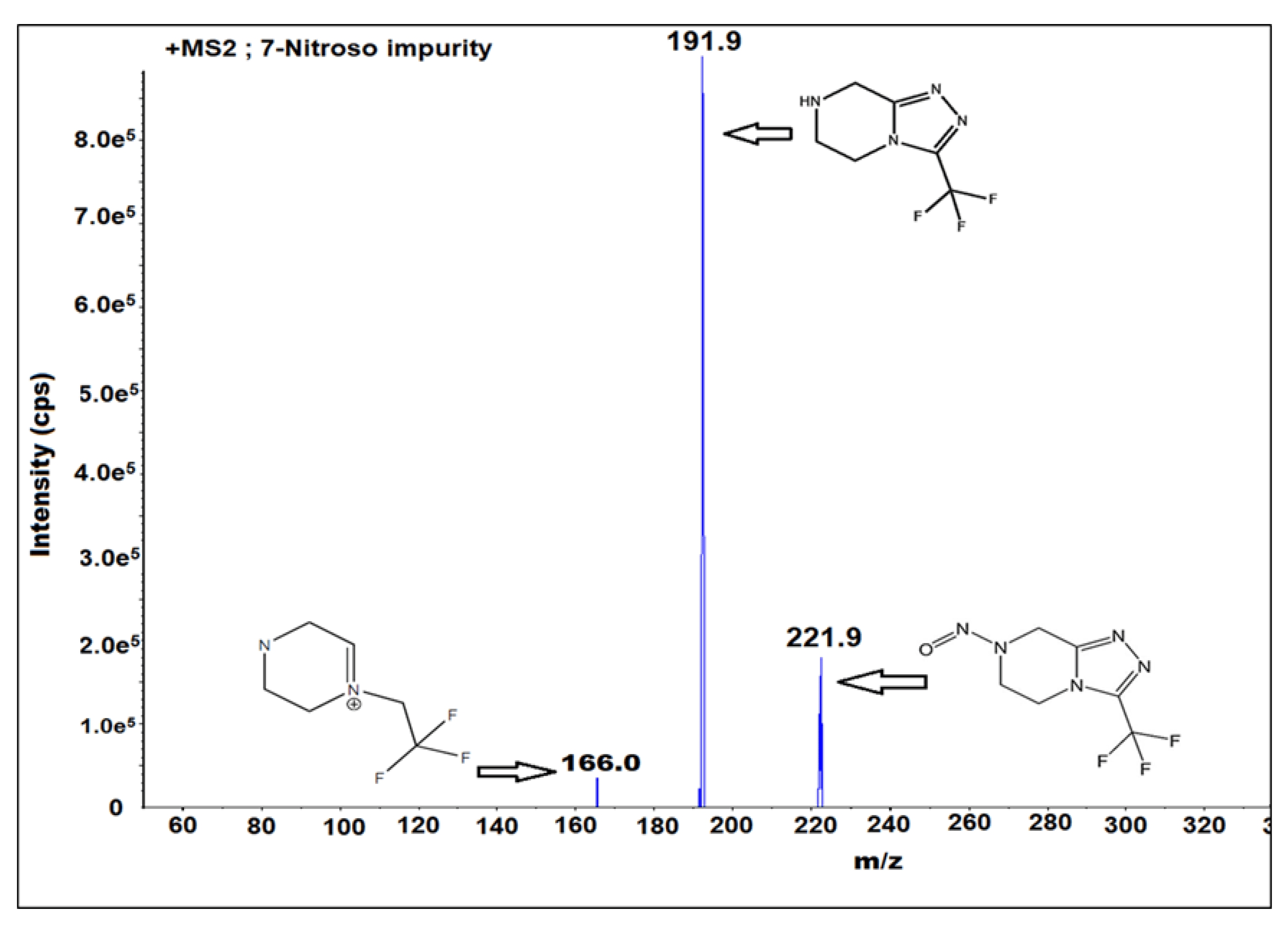
Chittireddy, H. N. P. R. et al. Molecules. 2022.
Case 2: Impurity quantification in salmon calcitonin using LC-MS, demonstrating the application of mass spectrometry quantification in biopharmaceutical quality control.
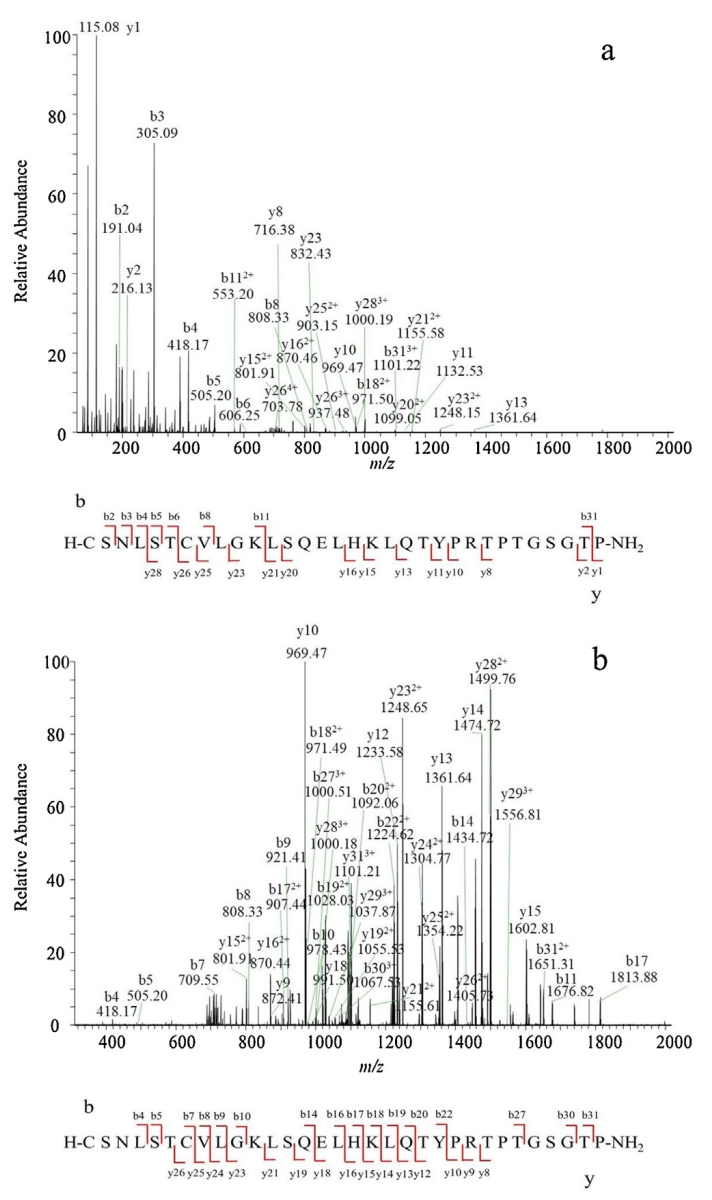
Wu, P. et al. J. Pharm. Biomed. Anal. 2020.
FAQ
Q1: How do you ensure the reliability of quantification results across multiple samples?
We implement rigorous experimental protocols and standardized analytical methods. For example, TMT and iTRAQ labeling technologies allow simultaneous quantification of up to 18 samples per experiment, minimizing inter-experiment error. Additionally, the use of internal standards and quality control samples ensures data consistency and reliability.
MtoZ Biolabs is committed to providing precise Mass Spectrometry Quantification Service, helping clients address impurity analysis challenges and ensuring products meet stringent quality standards. Contact us today to learn more!
What Could be Included in the Report?
1. Comprehensive Experimental Details
2. Materials, Instruments, and Methods
3. Total Ion Chromatogram & Quality Control Assessment
4. Data Analysis, Preprocessing, and Estimation
5. Bioinformatics Analysis
6. Raw Data Files
MtoZ Biolabs, an integrated chromatography and mass spectrometry (MS) services provider.
Related Services
Label-Free Quantitative Proteomics Service, MS Based
How to order?







