Label-Based Protein Quantitative Service—iTRAQ, TMT, SILAC
Dynamic changes of proteome abundance in cells significantly impact various life processes. For example, the onset and development of many diseases is often associated with the aberrant expression of certain proteins. Quantitative proteomics enables accurate quantification and identification of all proteins expressed within a genome or a complex mixture. Currently, quantitative proteomic techniques are primarily divided into labeling and label-free strategies, with labeling strategies further categorized into in vivo labeling (e.g., SILAC) and in vitro labeling (e.g., iTRAQ, TMT).
Isobaric Tags for Relative and Absolute Quantitation (iTRAQ)
iTRAQ is a quantitative proteomic technology developed by AB SCIEX in 2004. This technology can compare proteins across many different samples. For instance, it can examine differences in protein expression levels in tissue samples under various pathological conditions or developmental stages. At the same time, iTRAQ can accurately quantify and identify all proteins expressed in a genome or a complex mixture. iTRAQ can label and compare up to eight different protein samples simultaneously using eight different isotope. The labeling reagents consist of reporter group, balance group, and amine-specific reactive group.
Analysis Workflow of iTRAQ
The general process of iTRAQ is illustrated below. Briefly, a sample is cleaved by trypsin. The sample is then alkylated and digested into peptides by hydrolase. The peptides produced are then differentially labeled using iTRAQ reagents. The labeled samples are mixed and analyzed by LC-MS/MS.
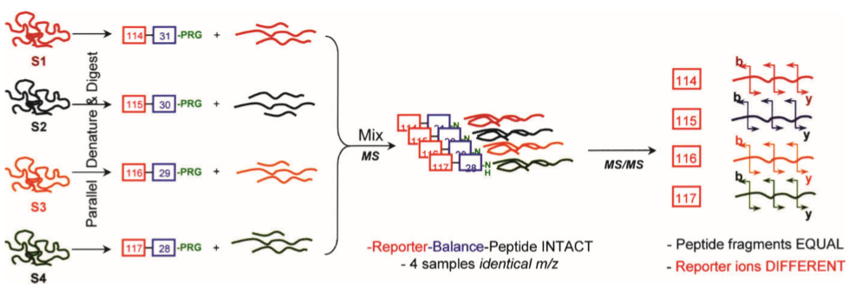
Figure 1. General Scheme of Multiple Reactions for Four Different Samples (S1-S4) Represented in Four Different Colors (Zieske et al., 2006)
Service Advantages of iTRAQ
1. In a single experiment, iTRAQ can simultaneously label 4 to 8 samples, providing a simple and fast operation. It is suitable for high-throughput detection of multiple samples, and its experimental design is more flexible.
2. iTRAQ is an in vitro labeling technique conducted at the peptide level and is suitable for many types of biological samples.
3. iTRAQ has good repeatability and high sensitivity, allowing simultaneous qualitative and quantitative analysis.
Tandem Mass Tags (TMT)
TMT is a peptide in vitro labeling technology developed by Thermo Fisher Scientific. This technology can use up to 10 isotopic tags to label the amino groups of peptides. After LC-MS/MS analysis, it can simultaneously compare the relative content of proteins across the 10 samples. TMT is widely used in fields such as disease biomarker screening, drug target identification, plant and animal disease- or stress-resistance mechanisms, plant and animal development and differentiation.
Analysis Workflow of TMT
The general process of TMT is illustrated below. Different protein samples are denatured, reduced, alkylated, and digested into peptides. These peptides are then labeled with a TMT kit and merged into a single sample. The merged sample is separated into fractions using HPRP liquid chromatography, and the fractions are then grouped into 12 samples. After purification, each sample is analyzed by HPLC-MS to gain protein qualitative and quantitative data.
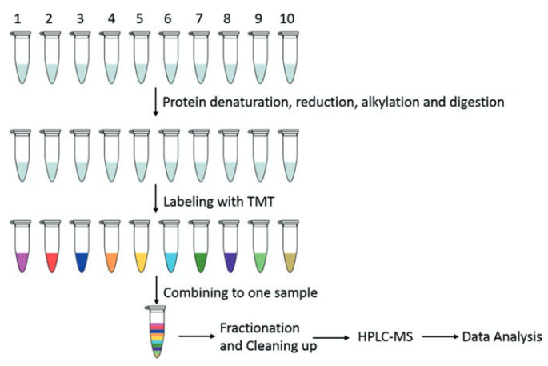
Figure 2. Workflow for Relative Quantification Using 10plex TMT (Zhang et al., 2017)
Service Advantages of TMT
1. High sensitivity: it can detect low abundance of proteins.
2. Wide separation range: it can separate acidic/basic proteins, proteins smaller than 10 kDa or larger than 200 kDa, and insoluble proteins.
3. Wide application range: it can identify any protein type, including membranous, nuclear, and extracellular proteins.
4. High throughput: it can analyze 10 samples simultaneously, making it especially suitable for differential protein analysis in samples with multiple treatments or time points.
Stable Isotope Labeling by Amino Acids in Cell Culture (SILAC)
SILAC is an effective scheme for high-throughput quantitative proteomics. It is designed based on the principle that mammalian cell proliferation depends on essential amino acids. SILAC can analyze protein interactions and expression differences in cells, providing an effective solution for comprehensive and systematic qualitative and quantitative proteomics analysis of complex mammalian cell.
Analysis Workflow of SILAC
The general process of SILAC is illustrated below. Essential amino acids (mainly lysine and arginine) labeled by light, medium, or heavy stable isotope are added to the culture medium. All newly produced proteins are labeled with stable isotopes through cellular metabolism. The proteins are mixed in equal amounts and separated by SDS-PAGE, followed by in-gel digestion and LC-MS/MS analysis to obtain quantitative and qualitative protein data.
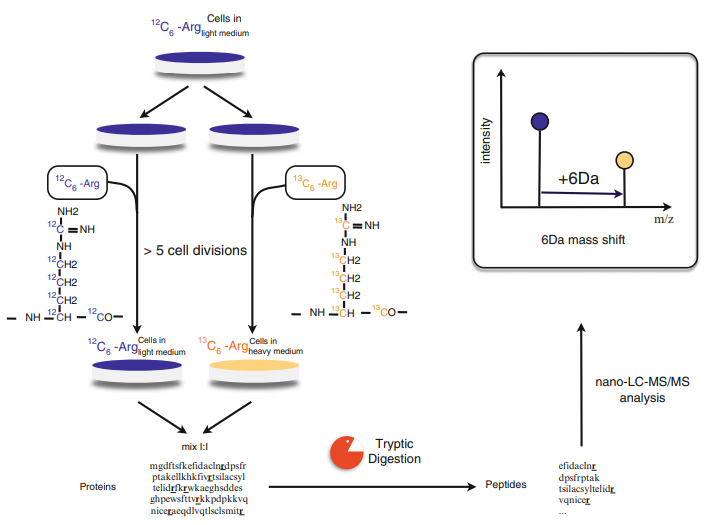 Figure 3. Protein Quantification Using SILAC (Esthelle et al., 2019)
Figure 3. Protein Quantification Using SILAC (Esthelle et al., 2019)
Service Advantages of SILAC
1. SILAC is a stable and effective labeling technology for live cell which is not affected by lysis buffer.
2. SILAC requires less sample amount with only tens of micrograms of protein needed per sample.
3. SILAC uses mass spectrometry to simultaneously identify and quantify multiple proteins.
4. SILAC employs in vivo labeling technology in which sample present in its approximately actual state.
MtoZ Biolabs, an integrated chromatography and mass spectrometry (MS) services provider.
Related Services
Quantitative Proteomics Service
How to order?







