Lipopolysaccharide Analysis Service
- Samples should be free from detergents and preservatives.
- Store samples at -80°C and ship with dry ice.
- Provide relevant details such as bacterial strain, growth medium, and experimental conditions.
MtoZ Biolabs provides comprehensive Lipopolysaccharide Analysis Service designed to support microbial pathogenesis research, vaccine formulation, and endotoxin quality control. Through the combination of advanced LC-MS/MS, GC-MS, and NMR technologies, we deliver detailed molecular information on bacterial LPS composition and structure. Our analytical expertise enables accurate interpretation of bacterial virulence factors, immune activation pathways, and contamination profiles.
Overview
Lipopolysaccharides, also known as endotoxins, are amphipathic macromolecules located in the outer membrane of Gram-negative bacteria. Each LPS molecule consists of three domains: lipid A, a core oligosaccharide, and an O-antigen polysaccharide chain. These components are essential for bacterial survival and play critical roles in immune recognition, inflammation, and antibiotic resistance.
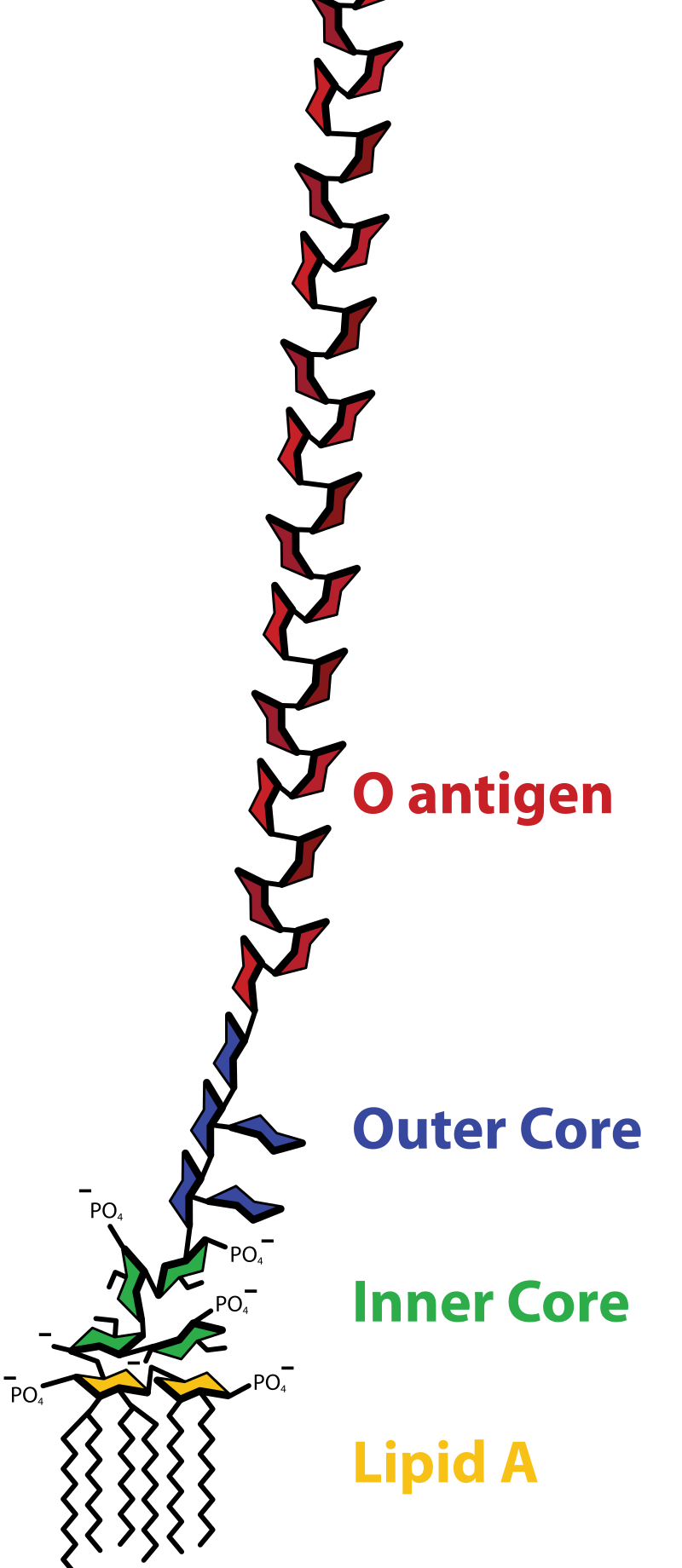
Figure 1. Structure of a Lipopolysaccharide (LPS)
By analyzing LPS composition and molecular architecture, researchers gain valuable insights into bacterial classification, pathogenicity, and host responses. Such information is crucial for developing novel vaccines, evaluating drug safety, and improving therapeutic design in both biomedical and industrial settings.
Services at MtoZ Biolabs
MtoZ Biolabs offers integrated Lipopolysaccharide Analysis Service covering compositional, structural, quantitative, and immunological evaluation of LPS. Each module is performed using validated chromatographic and spectrometric platforms to ensure data reliability and reproducibility.
Our main services include:
● Compositional Analysis of LPS Components
Comprehensive profiling of lipid A, core oligosaccharide, and O-antigen regions using LC-MS/MS and GC-MS. This service identifies molecular constituents and their relative abundance, providing a foundation for bacterial differentiation and strain comparison.
● Structural Elucidation of LPS Architecture
Detailed mapping of glycosidic linkages, acylation, and phosphorylation patterns through NMR and tandem MS. This analysis reveals molecular configurations and antigenic variations essential for understanding virulence and immune evasion mechanisms.
● Quantitative Endotoxin Determination
Accurate quantification of LPS concentration using LC-MS/MS or Limulus Amebocyte Lysate (LAL) assays. This workflow supports contamination monitoring, biopharmaceutical quality evaluation, and compliance with regulatory standards.
● Immunological Activity Evaluation
Functional assessment of LPS-induced immune responses through cytokine release tests, ELISA, and flow cytometry. These evaluations link molecular features with biological activity, offering deeper insight into immunogenicity and inflammatory potential.
All analyses are performed under strict quality control, with flexible project designs tailored to specific experimental objectives.
Sample Submission Suggestions
To ensure high-quality results, please prepare and submit samples according to the following guidelines:
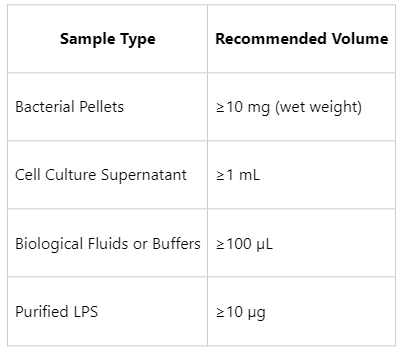
Additional Notes:
Our technical team provides personalized guidance for sample preparation, extraction, and compatibility assessment to achieve the best analytical outcomes.
Applications
The Lipopolysaccharide Analysis Service offered by MtoZ Biolabs supports a diverse range of research and industrial applications:
Vaccine Research and Development: Determining LPS structural diversity and immunogenicity to optimize vaccine formulations and adjuvant selection.
Endotoxin Monitoring and Quality Assurance: Measuring LPS contamination in pharmaceuticals, biologics, and culture media to ensure product safety and compliance.
Antibiotic Resistance Studies: Identifying structural modifications in lipid A and O-antigen regions associated with antimicrobial resistance.
Host–Pathogen Interaction Research: Exploring how LPS mediates immune signaling and inflammatory responses in mammalian hosts.
Bioprocess and Fermentation Optimization: Evaluating LPS synthesis and release during microbial growth and product manufacturing.
Food and Environmental Safety Testing: Detecting Gram-negative bacterial endotoxins in food, soil, and water samples for contamination assessment.
Why Choose MtoZ Biolabs?
✅ Comprehensive Analytical Capability: MtoZ Biolabs integrates lipidomics, glycomics, and proteomics expertise to deliver a complete and precise understanding of lipopolysaccharide structure and function.
✅ High-Resolution Instrumentation: Our advanced Orbitrap, timsTOF, and QTOF platforms provide accurate molecular characterization of lipid A, core sugars, and O-antigen variants.
✅ Reliable Endotoxin Quantification: Validated LC-MS/MS and LAL-based workflows ensure accurate endotoxin measurement for both research and quality control purposes.
✅ Broad Sample Compatibility: Our platform supports bacterial pellets, purified LPS, fermentation broths, and biological fluids, offering flexibility for various research needs.
✅ One-Time-Charge: Our pricing is transparent, no hidden fees or additional costs.
MtoZ Biolabs provides precise, reliable, and high-resolution Lipopolysaccharide Analysis Service for academic, pharmaceutical, and industrial applications. By integrating LC-MS/MS, GC-MS, and NMR-based profiling with expert interpretation, we deliver data-driven insights into the structure, function, and immunological significance of bacterial LPS. Partner with MtoZ Biolabs to accelerate your research and achieve dependable analytical outcomes.
How to order?







