Glycosylated Antibody Studies Service
MtoZ Biolabs offers Glycosylated Antibody Studies Service to provide comprehensive analysis of glycosylation patterns on therapeutic and research antibodies. Glycosylation is a critical post-translational modification (PTM) that significantly affects the structure, stability, and functionality of antibodies. By modifying the antibody’s Fc region, glycans influence various effector functions, including antibody-dependent cellular cytotoxicity (ADCC) and complement-dependent cytotoxicity (CDC), and also play a role in pharmacokinetics and immunogenicity.
Understanding the glycosylation profile of antibodies is essential for optimizing their therapeutic potential and ensuring product consistency. MtoZ Biolabs employs a range of advanced analytical techniques to provide accurate, reproducible data on glycosylation patterns, glycan diversity, and glycosylation site occupancy in monoclonal, polyclonal, and recombinant antibodies. This analysis is crucial for both research applications and biopharmaceutical development.
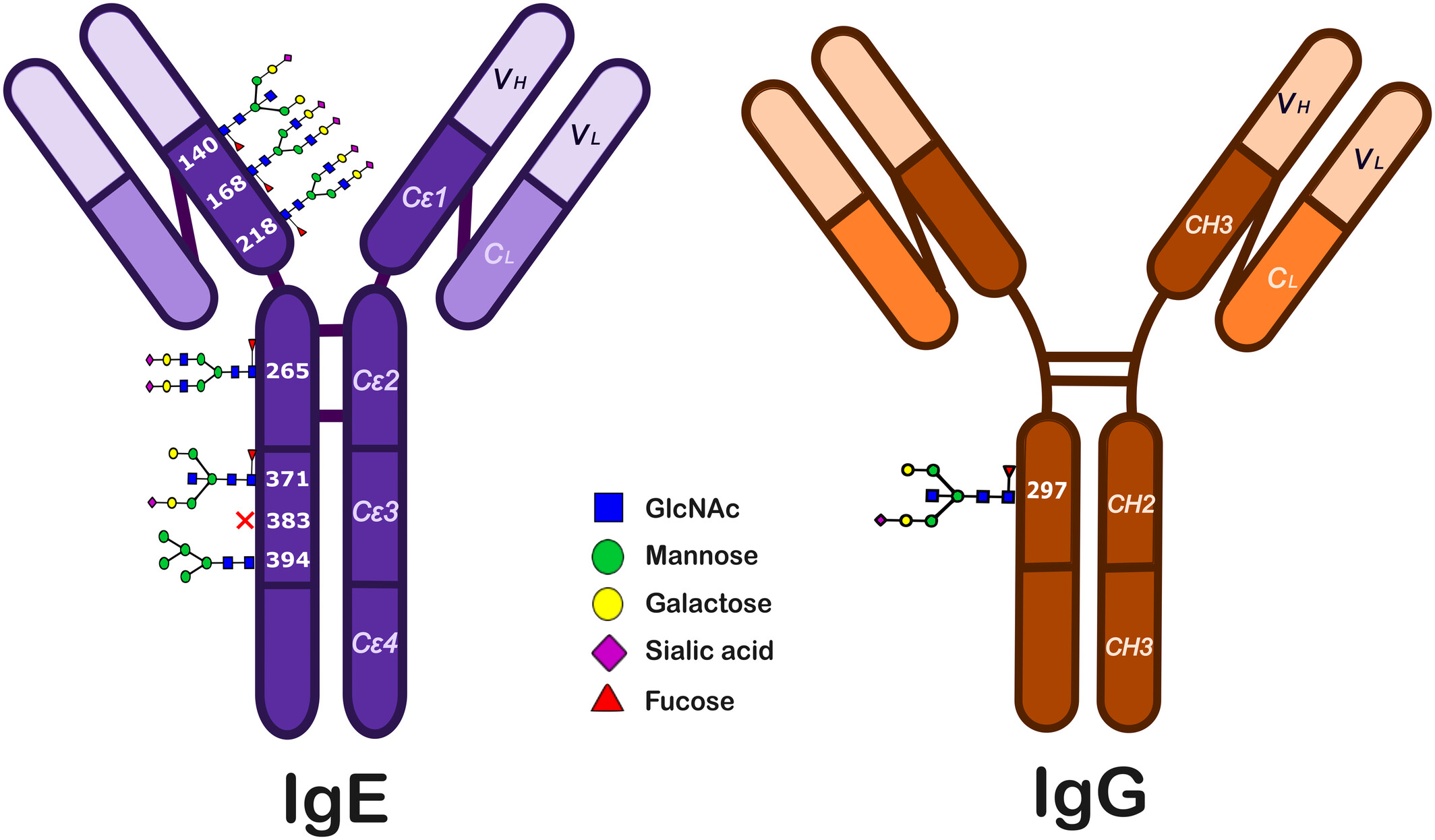
McCraw, A. J. et al. AACI. 2024.
Figure 1. Structures and Glycosylation Sites of IgE and IgG Class Antibodies
Services at MtoZ Biolabs
Our Glycosylated Antibody Studies Service is designed to support detailed analysis of glycan structures attached to antibodies, helping to identify key modifications and their functional implications.
1. Glycan Profiling
Using advanced liquid chromatography systems, MtoZ Biolabs separates and quantifies glycans attached to antibodies, providing insights into glycosylation diversity and profile. We employ various chromatography techniques, including HILIC and reverse-phase chromatography, to analyze complex glycan structures, including high-mannose, complex, and hybrid glycans.
2. Glycopeptide Mapping
Mass spectrometry is employed to analyze glycosylated peptides obtained from enzymatic digestion of antibodies. Using LC-MS/MS, we map the exact glycosylation sites, providing information on the heterogeneity and microheterogeneity of glycosylation. This method allows for high sensitivity and detailed glycosylation site characterization.
3. Glycoform Quantification
Quantifying the different glycoforms present in an antibody is crucial for assessing how variations in glycan structures impact antibody efficacy, stability, and pharmacokinetics. MtoZ Biolabs utilizes MS-based techniques, including label-free and isotopic labeling methods, to achieve precise quantification of glycoforms in a given antibody sample.
4. Glycosylation Site Occupancy
Through advanced analytical workflows combining enzymatic digestion, glycan release, and site-specific quantification, we determine the occupancy of glycosylation sites within antibodies. This analysis helps in understanding glycan attachment dynamics and optimizing antibody glycoengineering strategies.
5. Enzyme-Linked Lectin Assays (ELLA)
We employ lectin-based assays to evaluate the binding specificity of different glycans on antibodies. This technique is used to identify changes in glycosylation that may affect antibody function or interactions with receptors, enhancing the understanding of how glycan profiles influence immunological responses.
Sample Submission Suggestions
|
Sample Type |
Recommended Amount |
|
Antibody |
≥ 100 µg |
|
Cell Culture Supernatant |
≥ 1 mL |
|
Serum/Plasma |
≥ 100 µL |
|
Tissue |
≥ 20 mg |
Note: For optimal results, store samples at −80°C and ship on dry ice. Minimize freeze-thaw cycles to avoid degradation of antibodies. If you need further details, our technical support team is happy to assist and provide comprehensive guidance on sample submission.
Applications
Our Glycosylated Antibody Studies Service supports a wide range of applications across biopharmaceutical and research sectors:
Biopharmaceutical Development: Optimizing glycosylation patterns during antibody development to enhance therapeutic efficacy, improve pharmacokinetics, and reduce immunogenicity.
Vaccine Development: Understanding the glycosylation of antibodies used in vaccine formulations to ensure effective immune responses and to enhance vaccine stability and potency.
Bioprocessing and Quality Control: Analyzing glycosylation profiles of antibodies during manufacturing to ensure consistency, quality, and regulatory compliance.
Immunotherapy Optimization: Evaluating how glycosylation modifications affect the effector functions of therapeutic antibodies, such as ADCC and CDC, to optimize immunotherapies in cancer treatment and autoimmune diseases.
Research and Biomarker Discovery: Investigating the role of glycosylated antibodies in disease mechanisms and identifying novel biomarkers associated with glycosylation alterations in various diseases.
Why Choose MtoZ Biolabs?
· Advanced glycan profiling and glycopeptide mapping techniques
· High-sensitivity MS-based quantification of glycoforms
· Customizable analysis tailored to specific antibody types and applications
· Expertise in optimizing glycosylation for therapeutic antibodies
· Dedicated scientific support and consultation for antibody development and manufacturin
MtoZ Biolabs Glycosylated Antibody Studies Service offers essential insights into antibody glycosylation, enabling researchers and pharmaceutical companies to optimize antibody production, enhance therapeutic efficacy, and develop better-targeted treatments.
FAQ
1. How does glycosylation affect antibody functionality?
Glycosylation directly influences antibody activity by modulating its interaction with Fc receptors, stability, half-life, and immune system engagement. Variations in glycan structures can enhance or reduce antibody efficacy, particularly in immune response functions such as ADCC.
2. Can MtoZ Biolabs analyze glycosylation in different types of antibodies?
Yes, our Glycosylated Antibody Studies Service supports the analysis of various antibody formats, including monoclonal antibodies, polyclonal antibodies, and bispecific antibodies. We provide detailed glycosylation profiles for each type to support therapeutic development.
Related Services
How to order?







