DNA/RNA Drug Stability Analysis Service
- New Drug Development: Assess the stability of candidate drugs to guide molecule selection and formulation improvement.
- Degradation Pathway and Impurity Studies: Reveal potential degradation mechanisms and impurity profiles to ensure drug quality and safety throughout the entire lifecycle.
- Storage and Packaging Optimization: Provide scientific evidence for transportation conditions and packaging selection.
- Quality Control and Method Validation: Establish stability-indicating methods to ensure consistency.
- Shelf Life Prediction: Predict the shelf life of drugs under conventional conditions based on stability study data.
- Comprehensive Experimental Details
- Materials, Instruments, and Methods
- Total Ion Chromatogram & Quality Control Assessment (project-dependent)
- Data Analysis, Preprocessing, and Estimation (project-dependent)
- Bioinformatics Analysis
- Raw Data Files
DNA/RNA Drug Stability Analysis Service refers to the monitoring and characterization of nucleic acid drugs under controlled conditions, covering forced degradation studies, real-time stability assessments, and accelerated stability testing. Through multidimensional analytical techniques, this service comprehensively reveals the stability characteristics, potential impurity profiles, and changes in bioactivity of drugs, thereby providing reliable data support for research, manufacturing, and regulatory submission.
DNA and RNA drugs show great potential in therapeutic applications, but their molecular structures are highly sensitive to temperature, humidity, light, pH, and oxidative environments, making them prone to degradation or conformational changes that directly affect efficacy and safety. Systematic stability studies not only help to elucidate degradation pathways and impurity formation but also provide scientific evidence for shelf-life determination, formulation and packaging optimization, definition of transport and storage conditions, and regulatory compliance.
Services at MtoZ Biolabs
Relying on advanced platforms such as chromatography, mass spectrometry, electrophoresis, and spectroscopy, MtoZ Biolabs provides a DNA/RNA Drug Stability Analysis Service that comprehensively covers different stability study requirements.
1. DNA/RNA Drug Forced Degradation Analysis Service
Rapidly reveals potential degradation pathways and impurity formation of DNA or RNA drugs under extreme conditions.
2. DNA/RNA Drug Real-Time Stability Analysis Service
Monitors drug quality changes over time under actual storage conditions to support shelf-life determination and storage condition settings.
3. DNA/RNA Drug Accelerated Stability Analysis Service
Rapidly predict the long-term stability of drugs under accelerated conditions such as high temperature and high humidity, saving time for research and regulatory submission.
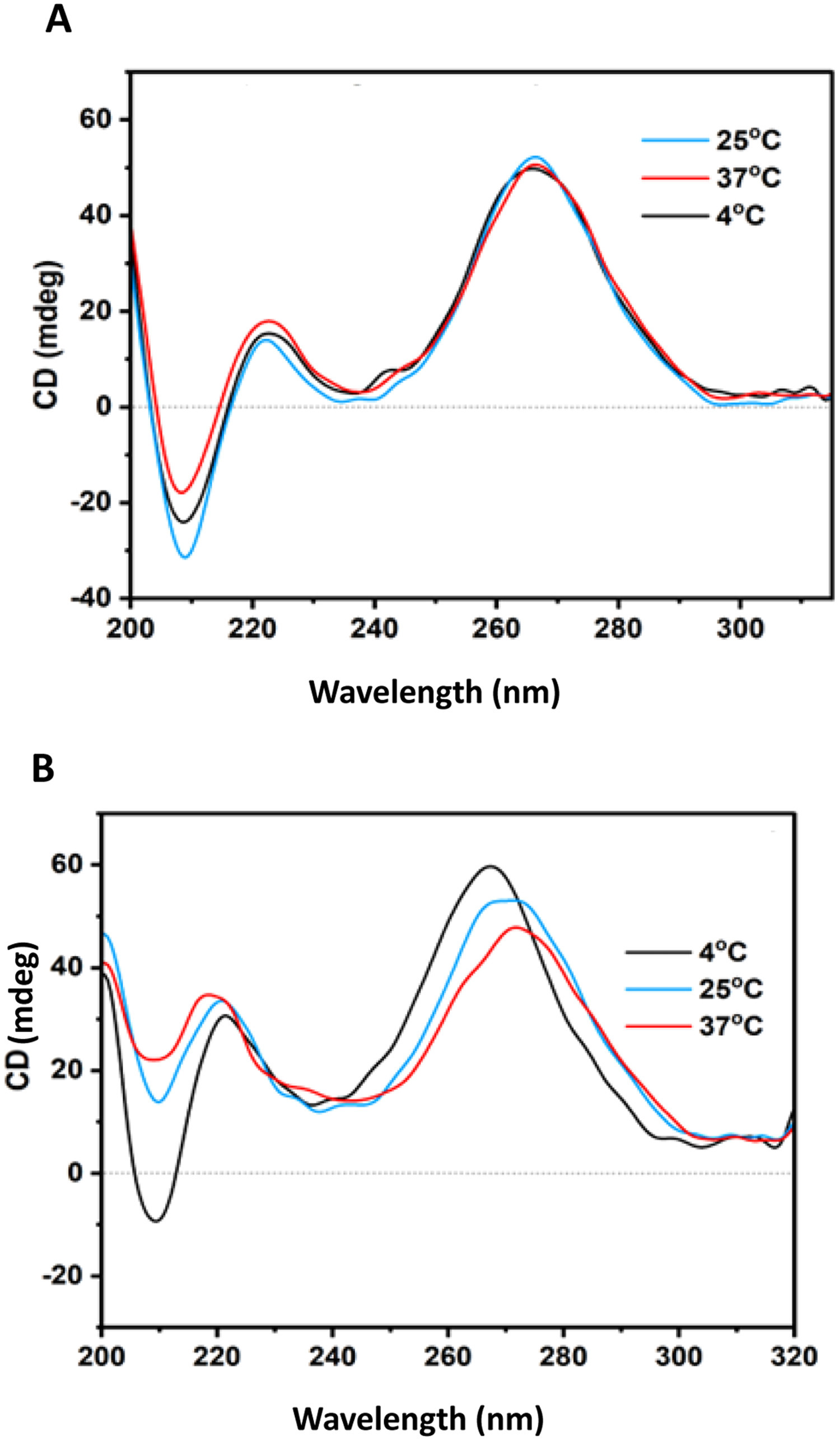
Chheda U. et al. J Pharm Sci. 2024.
Figure 1. Use Circular Dichroism to Detect the Effect of Temperature on MRNA Conformation
Analysis Workflow
The general workflow of the DNA/RNA Drug Stability Analysis Service is as follows:
1. Study Design
Set the experiment type (forced degradation, real-time stability, or accelerated stability), storage conditions, sampling schedule, and testing parameters based on drug characteristics and project requirements.
2. Sample Preparation
Prepare and preliminarily characterize DNA or RNA samples to establish a baseline control.
3. Experiment Execution
Place samples under the defined environmental conditions, perform sampling at scheduled time points, and conduct parameter testing.
4. Data Analysis
Integrate and analyze all data to evaluate changes in purity, degradation profiles, and maintenance of activity.
5. Report Generation
Deliver a complete experimental report including methods, raw data, stability assessment, and shelf-life prediction.
Sample Submission Suggestions
1. Sample Types
DNA, RNA, and their modified forms are accepted, including single-stranded, double-stranded, circular oligonucleotides, or mRNA.
2. Storage and Transportation
Samples are recommended to be stored at low temperatures of −20°C or below, transported on dry ice, and protected from repeated freeze–thaw cycles.
It is recommended to contact the MtoZ Biolabs technical team before sample submission to obtain detailed and tailored guidelines for sample preparation and submission.
Applications
Deliverables
How to order?







