DNA/RNA Drug Real-Time Stability Analysis Service
DNA/RNA Drug Real-Time Stability Analysis Service refers to the long-term monitoring and periodic testing of nucleic acid drugs under defined storage conditions such as ICH-recommended temperature, humidity, and light exposure, in order to evaluate their stability and degradation trends during actual storage and transportation. The goal of this service is to monitor in real time changes in purity, degradation products, and activity, to establish stability trend models, and ultimately to provide scientific evidence for setting drug shelf life, optimizing formulation and packaging, and supporting regulatory submissions.
DNA and RNA drugs, such as oligonucleotides, siRNA, mRNA vaccines, and aptamers, represent a new generation of therapeutic molecules with tremendous potential in clinical applications. However, these molecules are highly sensitive to temperature, humidity, light, pH, and nucleases, making them prone to degradation or conformational changes during storage and transport, which can compromise efficacy and safety. To ensure consistent quality and clinical effectiveness throughout their shelf life, Real-Time Stability Analysis has become an indispensable component of research and development, quality control, and regulatory compliance.
Services at MtoZ Biolabs
Relying on advanced platforms such as chromatography, mass spectrometry, UV/fluorescence spectroscopy, and functional activity assays, MtoZ Biolabs provides DNA/RNA Drug Real-Time Stability Analysis Service to systematically evaluate the stability of nucleic acid drugs under defined storage conditions and time intervals. Our service covers study design, sample storage management, periodic testing, data trend analysis, and stability report generation. Through the integration of multiple platforms and statistical modeling, MtoZ Biolabs delivers high-quality stability data and reliable shelf-life predictions, supporting drug development, formulation optimization, and regulatory submission.
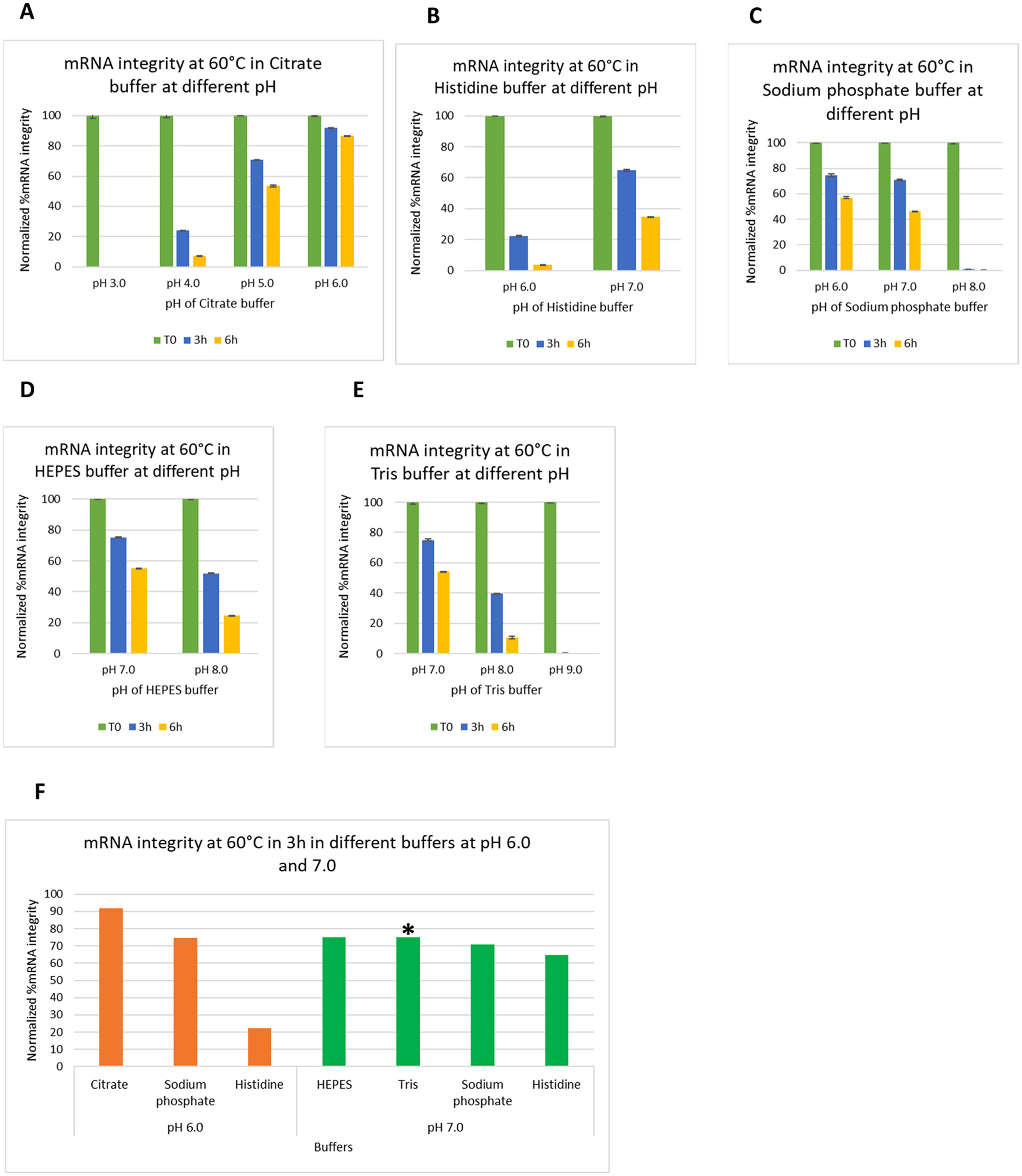
Chheda U. et al. J Pharm Sci. 2024.
Figure 1. Effect of buffering species and pH of the system on mRNA integrity.
Analysis Workflow
The general workflow of DNA/RNA Drug Real-Time Stability Analysis Service is as follows:
1. Study Design
Develop an experimental plan based on client requirements and drug characteristics, defining storage conditions, sampling time points, and testing parameters.
2. Sample Collection and Storage
Place samples in containers simulating actual packaging and store them under different conditions of temperature, humidity, and light exposure.
3. Stability Testing
Perform periodic sampling at predefined time points and use multiple platforms to test drug quality attributes.
4. Data Analysis
Identify degradation trends and changes in impurity profiles, and predict potential shelf life.
5. Report Generation
Deliver a complete stability study report including experimental data, statistical analysis, shelf-life prediction, and storage recommendations.
Service Advantages
1. Advanced Analysis Platform: MtoZ Biolabs established an advanced DNA/RNA Drug Real-Time Stability Analysis Service platform, guaranteeing reliable, fast, and highly accurate analysis service.
2. One-Time-Charge: Our pricing is transparent, no hidden fees or additional costs.
3. High-Data-Quality: Deep data coverage with strict data quality control. AI-powered bioinformatics platform integrates all DNA/RNA Drug Real-Time Stability Analysis data, providing clients with a comprehensive data report.
4. Customized Study Design: Develop personalized storage conditions and testing schedules based on drug properties and client requirements to meet different research and regulatory objectives.
5. Data Traceability and Security: Provide complete raw data and standardized reports while strictly enforcing confidentiality agreements to protect intellectual property and research outcomes.
Sample Submission Suggestions
Sample submission requirements are determined based on the specific project. It is recommended to contact the MtoZ Biolabs technical team prior to sample submission to obtain detailed and tailored guidelines for sample preparation and submission.
Applications
Drug Safety Evaluation: Conduct long-term monitoring of nucleic acid drug degradation under real storage conditions to ensure safety throughout the usage period.
Ensuring Drug Effectiveness: Confirm that nucleic acid drugs maintain activity and function during their shelf life.
Formulation and Packaging Optimization: Compare the effects of different buffers, excipients, or packaging materials on drug stability.
Shelf-Life and Expiration Date Setting: Use time-series data and trend analysis to predict drug expiration dates and support the establishment of appropriate shelf life and storage conditions.
FAQ
Q1: Which Analytical Methods Are Most Suitable for DNA/RNA Drugs?
A1: Liquid chromatography and capillary electrophoresis can be used to monitor purity and fragmentation, chromatography coupled with mass spectrometry can be applied for degradation product analysis, UV/fluorescence spectroscopy and circular dichroism can be used for conformational change monitoring, and functional activity assays can be included when necessary.
Q2: Can DNA/RNA Drug Real-Time Stability Analysis Service Detect Low-Abundance Degradation Products and Modification Loss?
A2: Yes. High-resolution mass spectrometry combined with sensitive separation methods can identify low-abundance impurities and modification loss, and kinetic analysis can be used to reveal potential degradation pathways.
Deliverables
1. Comprehensive Experimental Details
2. Materials, Instruments, and Methods
3. Total Ion Chromatogram & Quality Control Assessment (project-dependent)
4. Data Analysis, Preprocessing, and Estimation (project-dependent)
5. Bioinformatics Analysis
6. Raw Data Files
Related Services
DNA/RNA Drug Secondary Structures Analysis Service
DNA/RNA Drug Higher-Order Structure Analysis Service
DNA/RNA Drug Forced Degradation Analysis Service
How to order?







