DNA/RNA Drug Accelerated Stability Analysis Service
DNA/RNA Drug Accelerated Stability Analysis Service refers to the systematic storage and periodic testing of DNA or RNA drugs under controlled accelerated conditions, such as elevated temperature, high humidity, or light, combined with multiple analytical platforms including chromatography, mass spectrometry, electrophoresis, and spectroscopy to evaluate the purity, degradation products, modification status, and functional activity of the drug. Data obtained from accelerated experiments can be used to predict the stability performance of drugs under conventional conditions, providing scientific evidence for quality consistency evaluation, shelf-life prediction, and regulatory compliance.
Nucleic acid molecules are highly sensitive to temperature, humidity, light, pH, and oxidative conditions, making them prone to degradation or conformational changes that may compromise efficacy and safety. To predict the long-term stability of drugs under actual storage conditions within a shorter period of time, accelerated stability analysis has been widely applied in the research and regulatory submission of nucleic acid drugs, serving as an essential tool for shelf-life determination, formulation optimization, and packaging design improvement.
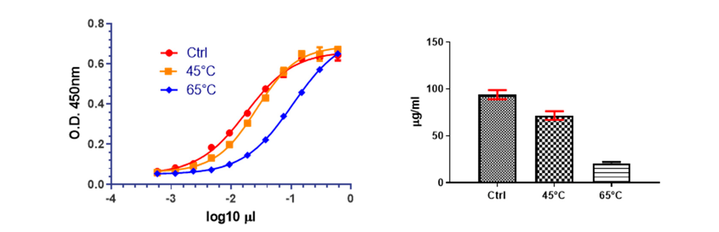
D'Alessio F. et al. Vaccines (Basel). 2023.
Figure 1. Characterization of DNA Accelerated Stability.
Services at MtoZ Biolabs
Relying on advanced analytical platforms such as chromatography, mass spectrometry, and spectroscopy, MtoZ Biolabs provides DNA/RNA Drug Accelerated Stability Analysis Service to systematically evaluate the stability of nucleic acid drugs under controlled conditions, including elevated temperature, high humidity, and light exposure. Our service covers study design, accelerated storage experiments, periodic testing, characterization of degradation products, and trend analysis. By extrapolating real shelf life from accelerated data, MtoZ Biolabs delivers comprehensive reports for stability evaluation, formulation and packaging optimization, and regulatory compliance, supporting drug development and regulatory submission.
Analysis Workflow
The general workflow of DNA/RNA Drug Accelerated Stability Analysis Service is as follows:
1. Study Design
An experimental plan is developed according to the characteristics of the drug and the research objectives, mainly including the setting of accelerated conditions such as elevated temperature, high humidity, and light exposure, as well as the determination of sampling time points and detection indicators.
2. Sample Preparation
DNA or RNA samples are prepared and characterized, and baseline data are recorded to serve as controls.
3. Accelerated Storage Experiment
Samples are stored under the defined accelerated conditions and are periodically collected under strict monitoring.
4. Quality Testing and Characterization
Multiple analytical methods are applied to assess the defined quality indicators of the samples.
5. Data Analysis
All data are comprehensively analyzed to evaluate stability trends and degradation pathways and to predict shelf life.
6. Report Generation
A complete experimental report is provided, including testing data, result interpretation, and recommendations for storage optimization.
Service Advantages
1. Advanced Analysis Platform
MtoZ Biolabs established an advanced DNA/RNA Drug Accelerated Stability Analysis Service platform, guaranteeing reliable, fast, and highly accurate analysis service.
2. One-Time-Charge
Our pricing is transparent, with no hidden fees or additional costs.
3. High-Data-Quality
Deep data coverage with strict data quality control. An AI-powered bioinformatics platform integrates all DNA/RNA Drug Accelerated Stability Analysis data, providing clients with a comprehensive data report.
4. Rapid Shelf-Life Prediction
Long-term stability is extrapolated from accelerated experiments to predict shelf life and storage conditions within a shorter time frame, thereby shortening the research and development cycle.
5. Customized Solutions
Personalized study designs are developed based on different types of DNA or RNA drugs and client requirements to meet multiple objectives, including research, quality control, and regulatory submission.
Sample Submission Suggestions
Sample submission requirements are determined based on the specific project. It is recommended to contact the MtoZ Biolabs technical team prior to sample submission to obtain detailed and tailored guidelines for sample preparation and submission.
Applications
1. Shelf-Life Prediction
Estimate the long-term stability of DNA or RNA drugs under conventional conditions to determine their expiration date.
2. Degradation Pathway and Impurity Profiling
Reveal the degradation mechanisms of nucleic acid drugs under different conditions and identify potential impurities or by-products.
3. Packaging and Storage Condition Evaluation
Compare the effects of different packaging materials and storage or transportation conditions on drug stability to optimize lifecycle management.
4. Regulatory Compliance and Registration Support
Provide stability data that meets international standards to support new drug registration and quality control systems.
FAQ
Q1: What Conditions Are Commonly Used in Accelerated Stability Studies?
A1: Typical conditions follow established guidelines, such as 40°C ± 2°C and 75% ± 5% RH, and additional stress tests including light exposure, pH variation, or oxidative stress may be applied depending on the characteristics of DNA or RNA drugs to simulate different degradation risks.
Q2: Can the Results of DNA/RNA Drug Accelerated Stability Analysis Service Be Used to Extrapolate Long-Term Stability?
A2: Long-term shelf life of 12 to 24 months or even longer can be extrapolated using trend modeling and statistical methods, with the scientific validity relying on the completeness of time-series data and methodological validation.
Deliverables
1. Comprehensive Experimental Details
2. Materials, Instruments, and Methods
3. Total Ion Chromatogram & Quality Control Assessment (project-dependent)
4. Data Analysis, Preprocessing, and Estimation (project-dependent)
5. Bioinformatics Analysis
6. Raw Data Files
Related Services
DNA/RNA Drug Forced Degradation Analysis Service
DNA/RNA Drug Real-Time Stability Analysis Service
DNA/RNA Drug Stability Analysis Service
How to order?







