Wheat Starch Analysis Service | Pharmaceutical Excipient
MtoZ Biolabs offers Wheat Starch Analysis Service, providing a comprehensive evaluation of wheat starch, including composition analysis, impurity profiling, physicochemical characterization, and functional performance testing. These results support pharmaceutical excipient quality control, formulation development, food industry innovation, and biomaterial applications. With advanced platforms and experienced scientists, we deliver accurate, reproducible, and compliant results.
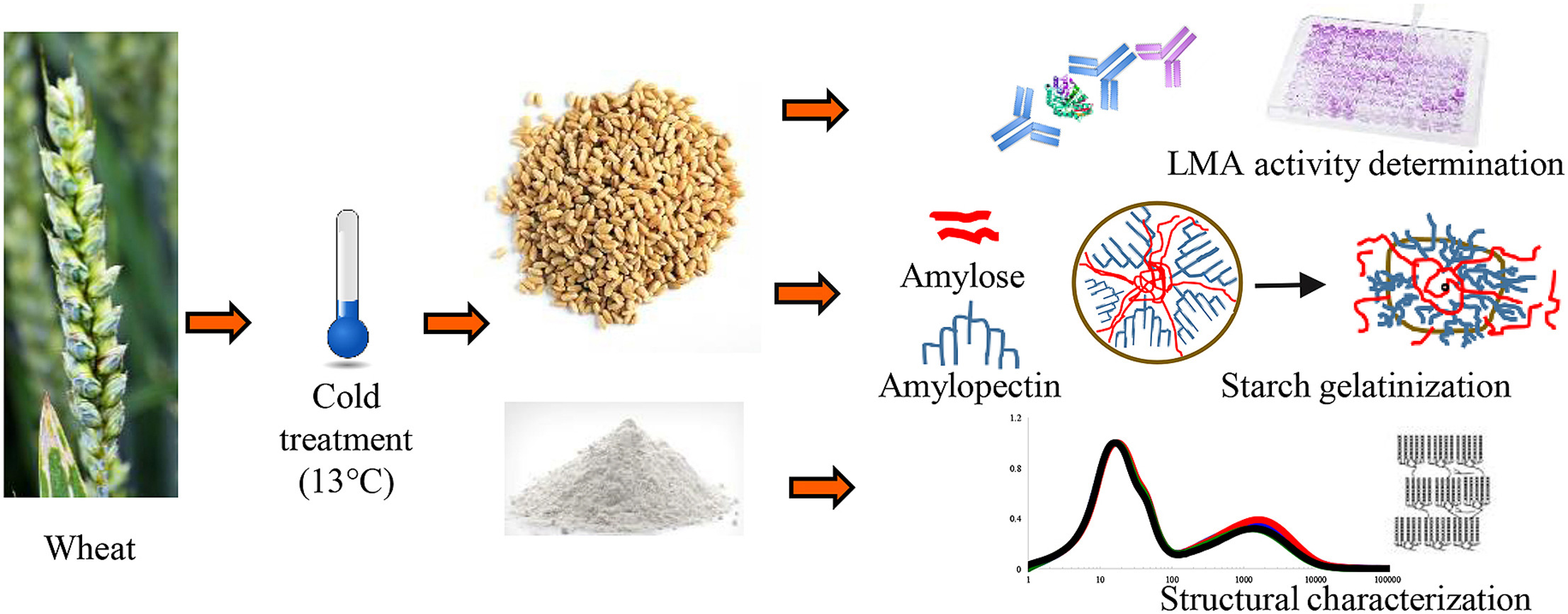
Wheat starch is a natural polysaccharide composed mainly of amylose and amylopectin, with particle sizes ranging from 10–60 μm for large granules to 2–10 μm for small granules. It features a fine texture and white appearance and is widely applied in pharmaceuticals, food, paper, fermentation, and materials science. In drug formulations, wheat starch functions as a diluent, disintegrant, and binder, directly influencing drug release and stability. Comprehensive analysis ensures excipient consistency and provides reliable data for formulation optimization and product innovation.
Neoh, G. K. S. et al. J. Cereal Sci. 2020.
Figure 1. Workflow of Wheat Starch Analysis
Services at MtoZ Biolabs
MtoZ Biolabs provides full-scale analytical solutions for wheat starch, from basic characterization to application-focused evaluations:
● Composition and Structural Characterization
Using microscopy, FTIR, NMR, and XRD, we determine amylose-to-amylopectin ratios, crystalline structure, and molecular characteristics, offering a clear view of starch composition and architecture.
● Purity and Quality Testing
HPLC, GC, and ICP-MS are applied to detect proteins, lipids, ash, and heavy metals. Additional parameters such as pH, sulfur oxide residues, oxidant levels, moisture content, and ash content are measured to verify compliance with pharmacopeial and food safety standards.
● Physicochemical Properties
Particle size distribution, solubility, gelatinization behavior, viscosity, and gel strength are analyzed to provide processing references for pharmaceutical and food applications.
● Microbiological Testing
Plate count, membrane filtration, and MPN methods are used to assess total bacterial and fungal counts, along with specified microorganisms, ensuring product safety and stability.
● Functional Performance Evaluation
In pharmaceuticals, we test disintegration and hygroscopicity. In food, we evaluate texture improvement, stability, and freeze-thaw resistance, linking laboratory analysis with real-world application.
Why Choose MtoZ Biolabs?
✅ Multi-Platform Analytical Capability: Equipped with HPLC, GC-MS, ICP-MS, FTIR, NMR, and DSC/TGA, enabling comprehensive testing of wheat starch from basic physicochemical properties to functional performance.
✅ Customized Testing Solutions: Flexible combinations of analytical methods are designed according to specific research goals and application needs, supporting excipient development, quality monitoring, and formulation optimization.
✅ Excipient-Specific Expertise: With extensive experience in pharmaceutical excipient research and testing, our team provides accurate data along with application-oriented insights to guide R&D and regulatory compliance.
✅ Safety and Quality Assurance: Testing emphasizes both physicochemical indicators and microbiological characteristics, helping clients evaluate the stability, safety, and reliability of wheat starch.
Sample Submission Suggestions
Accepted sample types for Wheat Starch Analysis Service include raw starch powders, pharmaceutical formulations, food samples, and industrial or biomaterial products. Specific requirements vary by type, but all samples should be securely sealed to prevent contamination and moisture. Powder samples are suitable for ambient shipping, while liquid or composite samples may require ambient or refrigerated transport depending on stability. Contact MtoZ Biolabs for detailed submission guidelines.
Applications
· Pharmaceutical Excipient QC: Evaluate batch consistency, disintegration, and compliance in tablets and capsules.
· Formulation Development: Study starch function as a matrix, binder, and disintegrant.
· Food and Beverage R&D: Test thickening, texture modification, and water retention performance.
· Industrial and Biomaterials: Explore potential in fermentation, biodegradable materials, and biopolymers.
Wheat starch is an important excipient and food ingredient, with quality and performance directly tied to product stability and market value. MtoZ Biolabs provides Wheat Starch Analysis Service with advanced instrumentation and expert interpretation, enabling clients to fully evaluate critical quality attributes. We also offer analytical services for other excipients such as hydroxypropyl methylcellulose, ethylcellulose, and microcrystalline cellulose, supporting formulation optimization, quality assurance, and regulatory compliance to accelerate product development from laboratory to market.
How to order?







