TMT Analysis Service
Tandem Mass Tags (TMT) technology, developed by Thermo Fisher, is an in vitro peptide labeling technique for quantitative analysis. TMT analysis service utilizes multiple stable isotope tags to specifically label the amine groups of peptides, enabling tandem mass spectrometry (MS/MS) to compare the relative abundance of proteins across up to 18 different samples simultaneously. This approach is widely used to study differences in protein expression levels in tissue samples under various pathological conditions or developmental stages.
The TMT tag consists of three components:
1. Reporter Group: Indicates the abundance level of protein samples.
2. Balance Group: Balances the mass of the reporter group to ensure identical total tag mass, guaranteeing that peptides labeled with the same tag exhibit identical m/z values.
3. Amine-Specific Reactive Group: Covalently binds to the N-terminus and lysine side-chain amines of peptides, enabling peptide labeling.
Peptides from different samples labeled with TMT reagents share the same mass and appear as a single mass spectrum peak (with identical retention time and m/z) during the first stage of mass spectrometry (MS1). When the selected spectrum peak undergoes fragmentation, the different reporter groups are released during the second stage of mass spectrometry (MS2). The signal intensity of each reporter ion peak corresponds to the relative abundance of the peptide in the respective sample.
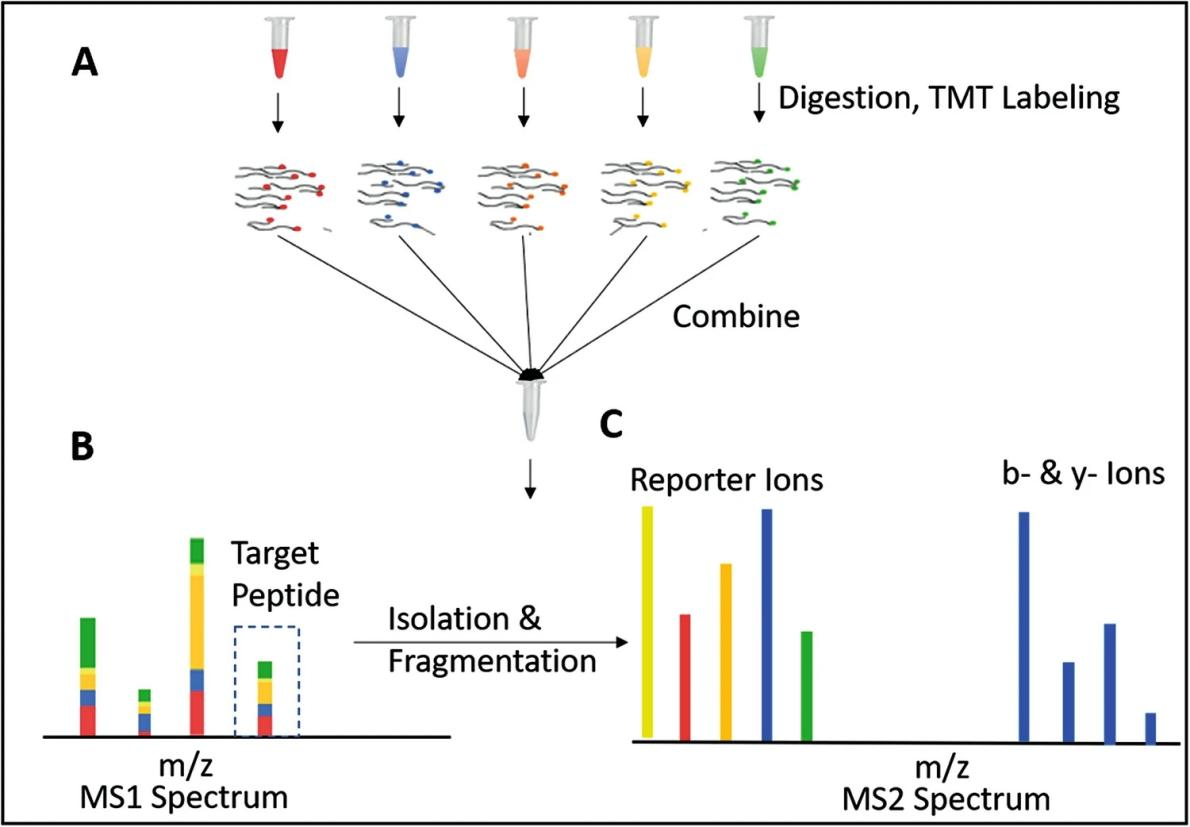
Gupta, Sonnett. et al. Xenopus: Methods and Protocols, 2018.
Figure 1. Principle of TMT Labeling Quantification.
Services at MtoZ Biolabs
MtoZ Biolabs has established TMT analysis service platform using Thermo Fisher's Orbitrap Fusion Lumos mass spectrometry system coupled with nanoLC-MS/MS nano-liquid chromatography. This platform provides researchers with highly sensitive and high-resolution quantitative proteomics solutions. The Orbitrap Fusion Lumos is one of the highest-resolution and most sensitive mass spectrometers available today, ensuring precise identification of low-abundance peptide fragments. Additionally, the use of HCD and ETD fragmentation modes guarantees the integrity of peptide fragments during analysis.
Service Advantages
1. High Throughput and Professional Testing
The TMT analysis service can simultaneously label up to 18 samples, significantly improving experimental efficiency. Expert personnel perform the experiments, ensuring stable operations and shortening data delivery timelines.
2. High-Quality Data
The service employs fractionation of labeled samples, resulting in deeper data coverage. With professional operation and the TMT labeling method, it ensures accurate quantification and provides comprehensive data analysis.
3. Excellent Service
MtoZ Biolabs offers customized pre-sales consultations, post-sales data interpretation, and project revision services. We promise unlimited support for both pre-sales and post-sales services without additional charges.
4. Transparent Pricing
Our pricing is transparent, with no hidden or extra fees.
Sample Submission Suggestions
MtoZ Biolabs provides TMT analysis service that can process samples in both liquid and solid states. For solid samples, transportation with ice packs is sufficient. For liquid samples, they can be vacuum-dried or freeze-dried and then transported with ice packs or dry ice to ensure sample integrity during transit.
Applications
TMT labeling technology is widely used in differential expression proteomics and the discovery of disease protein biomarkers. It is particularly suited for quantitative comparisons of proteins in complex biological samples, playing a crucial role in disease mechanism research, biomarker discovery, and drug action mechanism analysis.
1. Disease Mechanism Research
TMT labeling methods analyze dynamic protein expression changes in disease states, unveiling molecular mechanisms underlying disease progression.
2. Drug Development
TMT analysis service supports studying proteomic changes before and after drug treatment, aiding drug target validation and therapeutic effect evaluation.
3. Basic Scientific Research
TMT labeling explores fundamental biological questions, including cell signaling pathways and metabolic regulation networks.
4. Agriculture, Forestry, and Livestock
TMT analysis service can investigate stress tolerance mechanisms, growth and development, breeding protection, meat and milk quality, and disease mechanisms.
5. Marine and Aquaculture Sciences
TMT technology is utilized to study fisheries resources, aquaculture, and seafood safety and environmental conditions.
6. Food and Nutrition
TMT labeling facilitates studies on food storage and processing optimization, ingredient and quality identification, and functional food development.
Case Study
1. Using TMT Labeling to Investigate the Relationship Between Neutrophil Degranulation and Mortality in Alcohol-Associated Hepatitis Patients
The mortality rate among patients with alcohol-associated hepatitis (AH) is significantly high. Alcohol exacerbates liver damage by inducing gut dysbiosis, bacterial translocation, and inflammation, characterized by an increased number of neutrophils in the liver. This study utilized TMT labeling to analyze fecal proteins from multi-center cohorts comprising control groups, patients with alcohol use disorder (AUD), and AH patients. Reactome pathway and gene ontology analyses were employed to identify proteins with differential abundance across groups, pinpointing those most affected. In a second independent multicenter cohort (AlcHepNet), fecal biomarkers and their prognostic value were validated using ELISA in AH patient samples. The results revealed significant differences in the fecal proteomic profiles of the control, AUD, and AH groups. Proteins showing distinct abundance patterns across the three groups increased progressively with the severity of alcohol-associated liver disease and were predominantly localized to neutrophil granules. Further analysis confirmed that differentially regulated proteins were part of the neutrophil granule and degranulation pathways. Myeloperoxidase (MPO), a marker protein of neutrophil granules, was associated with disease severity and could predict 60-day mortality. An independent validation cohort confirmed that fecal MPO levels were predictive of short-term survival over 60 days. In summary, the increased abundance of proteins related to neutrophil degranulation in the feces of AH patients predicts short-term survival and serves as a non-invasive prognostic biomarker.
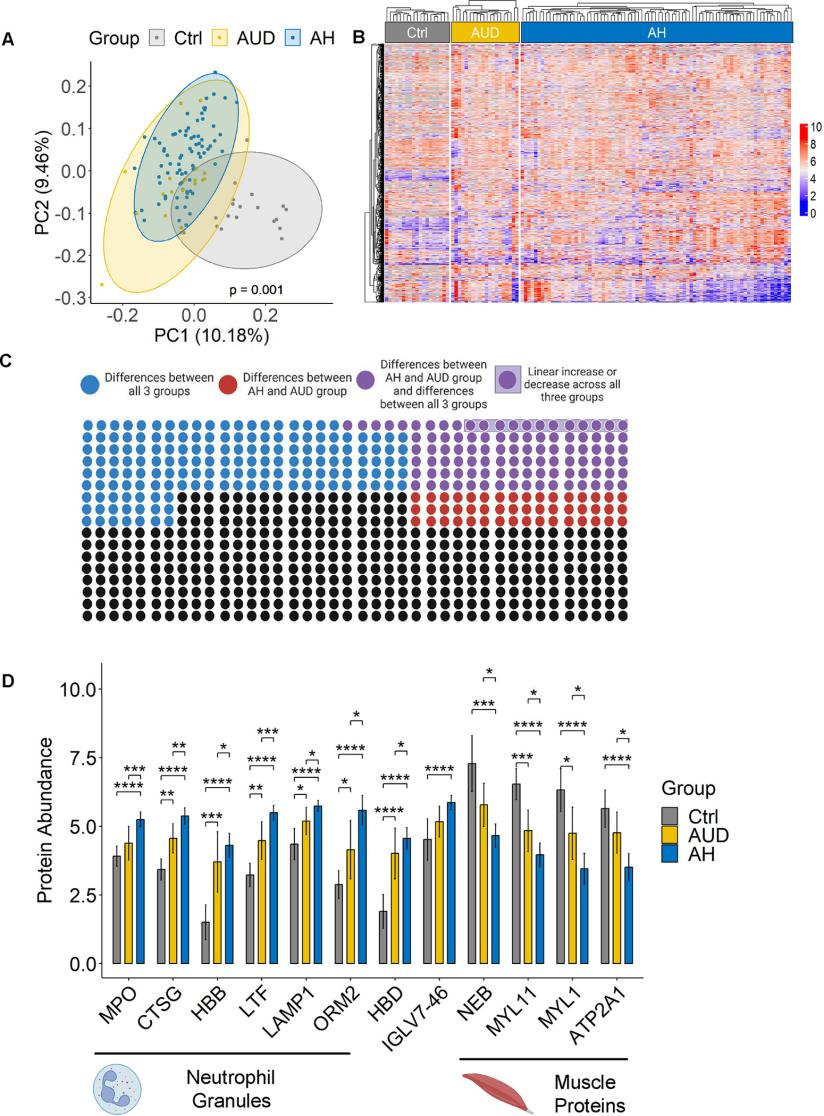
Kreimeyer, Gonzalez et al. Gut.2024
Figure 2. Fecal Protein Patterns Differ between Controls, Patients with Alcohol Use Disorder (AUD), and Patients with Alcohol-Associated Hepatitis (AH).
2. Quantitative Proteomics Analysis Using TMT Labeling to Investigate Protein Changes in Lamb Meat Under Different Feeding Conditions and Their Relationship to Flavor
In order to explore the molecular mechanism of the effect of feeding methods on mutton flavor, the biceps femoris samples of sheep in the grazing group (PF) and the concentrate feeding group (CF) were selected, mass spectrometry (MS) combined with TMT analysis service was used to investigate the relationship between flavor metrics and proteome profiles. Compared to the CF group, the PF group exhibited superior amino acid and volatile flavor compound content and composition. Beneficial flavor components such as arginine, pentanal, heptanal, octanal, 1-octen-3-ol, and 2,3-octanedione were found in higher concentrations in the PF group. A total of 82 significantly differentially abundant proteins (DAPs) were identified. GO and KEGG analyses revealed that pathways related to lamb flavor were primarily concentrated in amino acid synthesis and metabolism. These findings provide a foundation for further understanding the molecular mechanisms by which proteins regulate meat flavor.
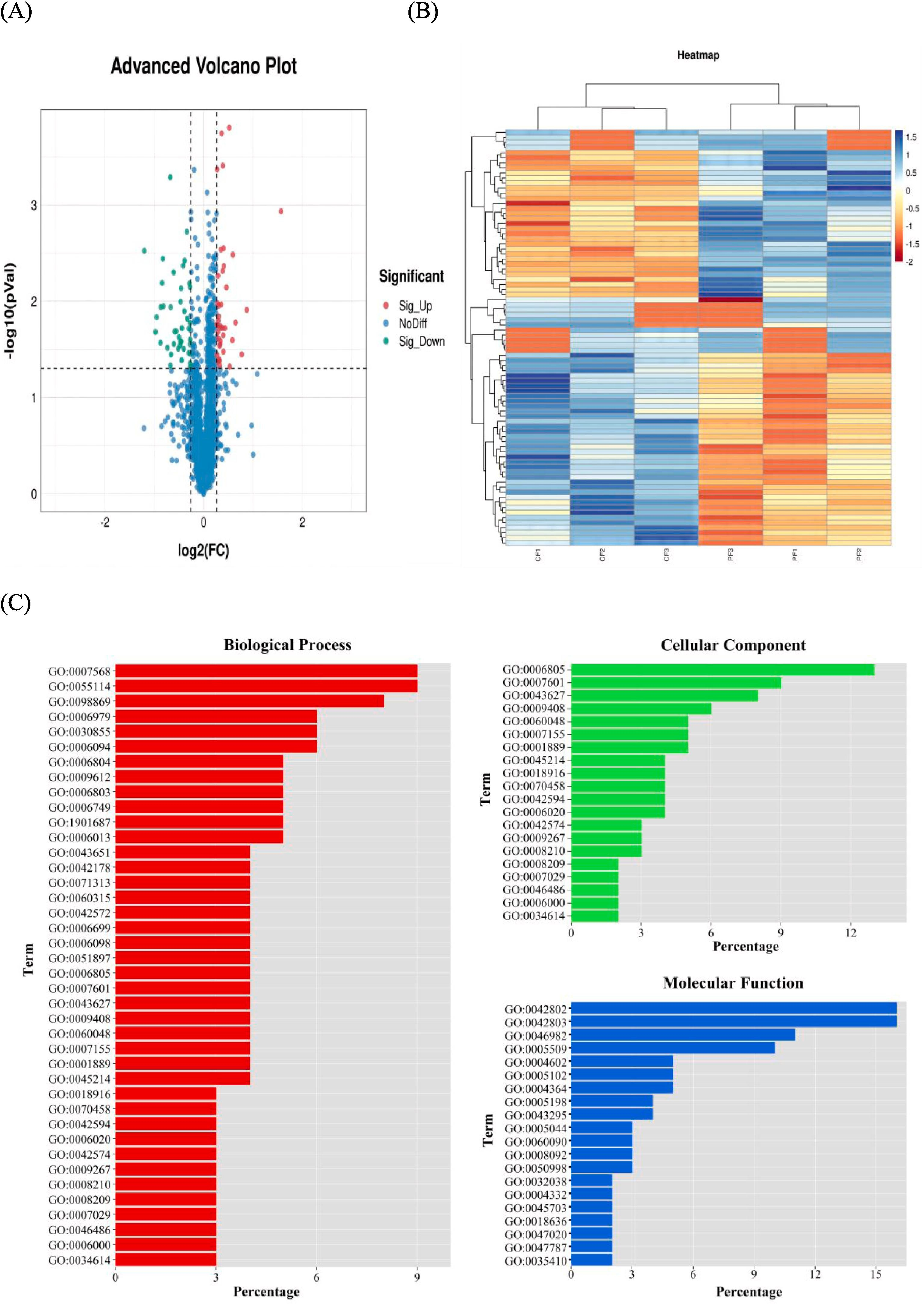
Yang, Hou. et al. Food Chemistry, 2024.
Figure 3. Cluster Analysis of Gene Expression Profiles.
Deliverables
1. Comprehensive Experimental Details
2. Materials, Instruments, and Methods
3. Total Ion Chromatogram & Quality Control Assessment
4. Data Analysis, Preprocessing, and Estimation
5. Bioinformatics Analysis
6. Raw Data Files
MtoZ Biolabs, an integrated Chromatography and Mass Spectrometry (MS) Services Provider, provides advanced proteomics, metabolomics, and biopharmaceutical analysis services to researchers in biochemistry, biotechnology, and biopharmaceutical fields. Our ultimate aim is to provide more rapid, high-throughput, and cost-effective analysis, with exceptional data quality and minimal sample consumption. Free project evaluation, welcome to learn more details!
MtoZ Biolabs, an integrated chromatography and mass spectrometry (MS) services provider.
Related Services
Quantitative Proteomics Service
iTRAQ-based Quantitative Proteomics Analysis
SILAC (Stable Isotope Labeling by Amino Acids in Cell Culture)-Based Quantitative Service
How to order?







