Tapioca Starch Analysis Service | Pharmaceutical Excipient
MtoZ Biolabs provides Tapioca Starch Analysis Service with a focus on systematic testing and quality evaluation of pharmaceutical excipient tapioca starch. Sourced from cassava roots, tapioca starch is valued for its good flowability, low allergenicity, and excellent gelatinization behavior. It is widely used in tablets as a diluent, binder, and disintegrant. Modified tapioca starch, known for its emulsifying, thickening, and film-forming properties, is also indispensable in food, textile, and paper industries. Because its physicochemical properties directly influence drug formulation performance, comprehensive analysis is critical for pharmaceutical development, excipient compatibility, and industrial process optimization.
Chamorro, A. F. et al. Polymers. 2025.
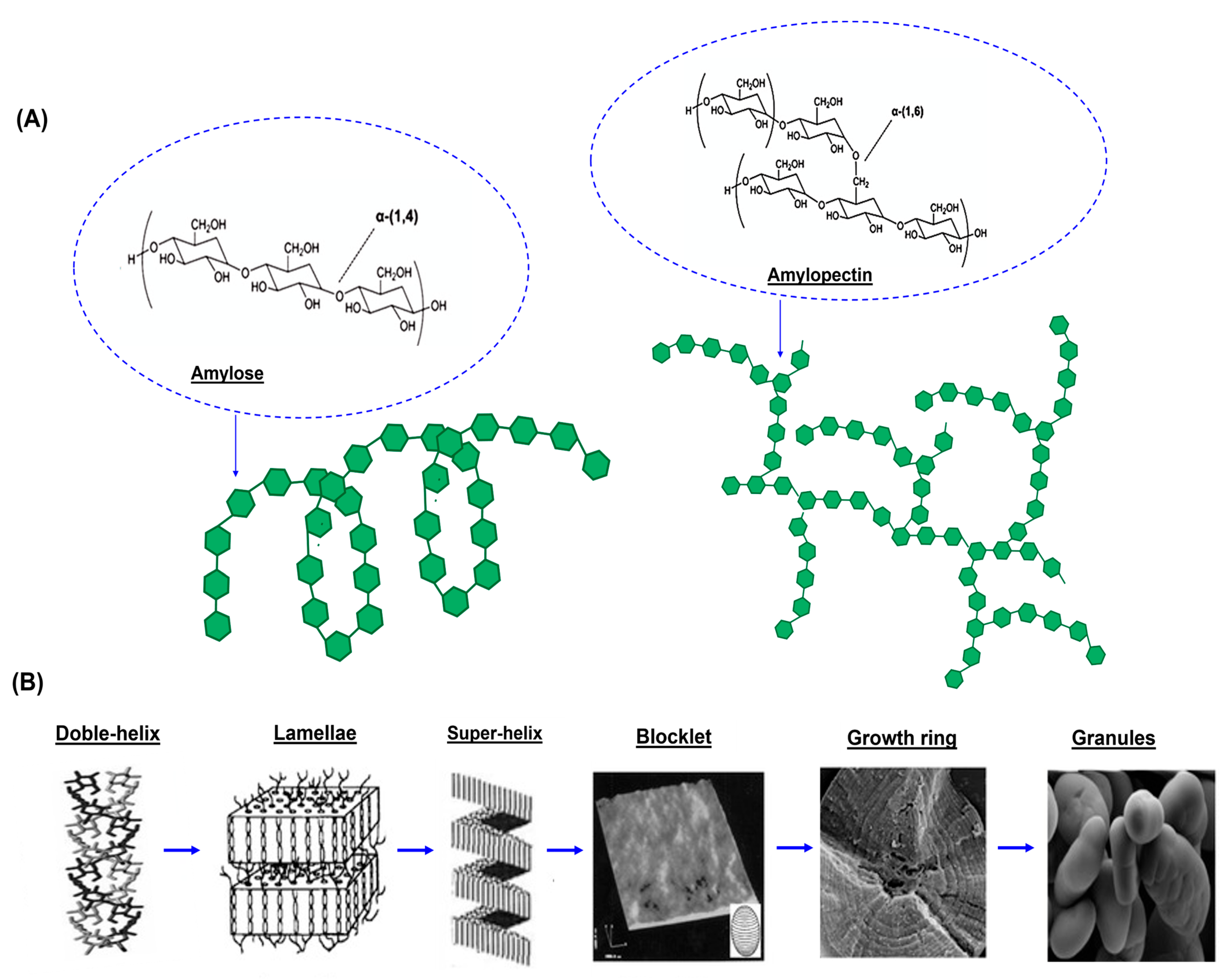
Figure 1. Structure of Tapioca Starch
With advanced analytical platforms and an experienced scientific team, MtoZ Biolabs offers standardized and customized solutions to support pharmaceutical companies, research institutes, and related industries, ensuring product safety, stability, and regulatory compliance.
Services at MtoZ Biolabs
MtoZ Biolabs offers a systematic testing program for tapioca starch, integrating both physical and chemical characterization to deliver a full picture of quality and functionality.
● Microscopic Characterization
Optical and polarized light microscopy are used to observe particle morphology (5–35 μm) and birefringence patterns, confirming starch identity and granule integrity.
● Particle Size Distribution
Laser diffraction analysis evaluates particle size and uniformity, providing essential data for consistent processing and formulation stability.
● Gelatinization and Rheological Behavior
Differential scanning calorimetry (DSC) and rotational rheometry measure gelatinization temperature, viscosity, and thermal stability, predicting excipient performance in formulations.
● Moisture and Loss on Drying
Gravimetric and thermal analysis (TGA) assess water content and stability, supporting safe storage and handling.
● PH and Amylose/Amylopectin Ratio
pH is measured to confirm batch consistency (range 4.5–7.0), while iodine tests and spectrophotometry reveal structural composition of starch.
● Residues and Impurities
Sulfur dioxide residues are quantified by titration, oxidative impurities are assessed by iodometry with UV-Vis detection, and iron salts are measured with ICP-MS. These parameters ensure compliance with safety standards.
● Microbiological Safety
Plate counts and specific organism tests monitor microbial limits, including E. coli, ensuring safe application in pharmaceuticals and foods.
Chamorro, A. F. et al. Polymers. 2025.
Why Choose MtoZ Biolabs?
✅ Advanced Instrumentation: Comprehensive coverage including microscopy, rheology, thermal analysis, chromatography, and mass spectrometry.
✅ Systematic Evaluation: Simultaneous assessment of structure, physicochemical traits, and safety.
✅ Customizable Solutions: Flexible test combinations tailored for R&D, QC, or regulatory submissions.
✅ Expert Interpretation: Reports combine accurate data with practical insights for real-world application.
✅ One-Time-Charge: Our pricing is transparent, no hidden fees or additional costs.
Sample Submission Suggestions
Our Tapioca Starch Analysis Service accepts raw starch powders, pharmaceutical formulations, food samples, and industrial or biomaterial products. Submission requirements vary by type; detailed guidelines are available upon request. Samples must be sealed to prevent contamination and moisture. Powder samples are generally suitable for ambient shipping, while liquid or composite samples may require ambient or cold transport depending on stability.
Applications
· Pharmaceutical Development and Quality Control: Evaluate excipient compatibility, disintegration, and binding performance.
· Food Industry Optimization: Support product development with modified starch for thickening, stabilization, and texture modification.
· Industrial Process Improvement: Facilitate applications in textiles, papermaking, fermentation, and biodegradable materials.
Tapioca starch plays a critical role in pharmaceuticals, foods, and industrial applications. MtoZ Biolabs provides Tapioca Starch Analysis Service with high standards of accuracy and reproducibility, ensuring that clients can fully understand the quality and suitability of this excipient. Beyond tapioca starch, our services also cover corn, potato, and wheat starch, delivering comprehensive excipient testing solutions to support formulation optimization, regulatory compliance, and industrial innovation.
Related Services
How to order?







