Sodium Starch Phosphate Analysis Service | Pharmaceutical Excipient
- Sample Types: Powdered SSP is preferred. For solutions, please indicate concentration and solvent.
- Storage and Transportation: Samples should be sealed and stored under dry, light-protected conditions. Low temperature or dry ice shipping is recommended.
Sodium Starch Phosphate Analysis Service is a systematic analytical service for evaluating the composition, content, solubility, stability, and other key properties of sodium starch phosphate. The service aims to comprehensively characterize its functional properties as a pharmaceutical excipient and provide reliable data to support drug research and manufacturing.
Sodium starch phosphate (SSP) is a commonly used modified starch in which phosphate groups are introduced into the starch molecule to enhance its stability, flowability, and disintegration performance, and it is widely applied as an excipient, disintegrant, and binder in pharmaceutical formulations. As a pharmaceutical excipient, the performance of SSP directly influences tablet hardness, dissolution behavior, and storage stability. Therefore, systematic analysis of the composition, structure, thermal behavior, and functional characteristics of SSP is of great importance for ensuring drug quality consistency, optimizing formulation processes, and meeting regulatory requirements.
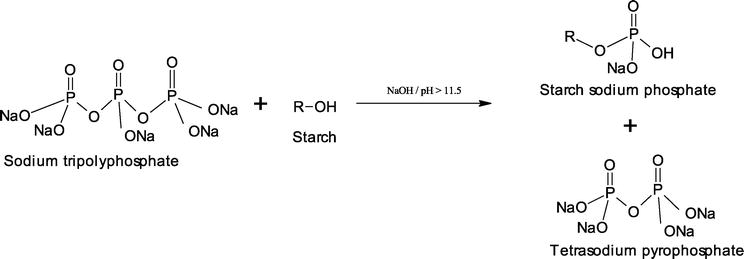
Egharevba HO. Chemical Properties of Starch and Its Application in the Food Industry. 2019.
Figure 1. Phosphorylation of Starch with Sodium Tripolyphosphate
Services at MtoZ Biolabs
MtoZ Biolabs provides a comprehensive Sodium Starch Phosphate Analysis Service to systematically evaluate the physicochemical properties and functional performance of sodium starch phosphate.
1. Purity and Content Testing
High-performance liquid chromatography (HPLC) and spectroscopic methods are applied to accurately determine the content of sodium starch phosphate and detect potential impurities.
2. Solubility Evaluation
In vitro solubility experiments and dissolution behavior tests are conducted to study its solubility characteristics in different solvents and pharmaceutical formulation environments, supporting formulation design and optimization.
3. Stability Analysis
Differential scanning calorimetry (DSC), thermogravimetric analysis (TGA), and spectroscopic techniques are used to assess the stability and degradation behavior of SSP under conditions of temperature, humidity, and light exposure.
4. Microbial Contamination Testing
Microbial culture and enumeration methods are employed to detect and identify bacterial, mold, and yeast contamination levels, providing targeted prevention and control recommendations to ensure the safety of the excipient.
Service Advantages
1. Advanced Analysis Platform
MtoZ Biolabs established an advanced Sodium Starch Phosphate Analysis Service platform, guaranteeing reliable, fast, and highly accurate analysis service.
2. Multidimensional Comprehensive Analysis
Provide full-scale testing from purity, content, solubility, and thermal stability to microbiological safety.
3. Customized Solutions
Flexibly select testing items and depth according to client requirements.
Sample Submission Suggestions
It is recommended to contact the MtoZ Biolabs technical team prior to sample submission to obtain detailed and tailored guidelines for sample preparation and submission.
Applications
1. Formulation Optimization
By analyzing the purity, solubility, and disintegration properties of SSP, formulation design can be optimized to improve drug disintegration rate and dissolution behavior.
2. Quality Control
Monitor the consistency of different batches of excipients in compliance with the pharmacopeia and regulatory standards.
3. Stability Studies
Evaluate the stability of SSP under conditions of temperature, humidity, and light, providing guidance for storage settings and shelf-life prediction.
4. Drug Delivery Research
Verify the applicability of SSP in sustained-release or controlled-release formulations, supporting the development of novel drug delivery systems.
How to order?







