Sodium Starch Glycolate Analysis Service | Pharmaceutical Excipient
- Sample Reception and Registration: Receive SSG powder samples and record sample information.
- Sample Preparation: Subsampling, drying, or dissolution based on analysis requirements.
- Physical Property Testing: Measure appearance, flowability, particle size, and water absorption.
- Chemical and Structural Analysis: Confirm molecular structure using FTIR and XRD, determine solubility and DS value, and detect residual chloroacetic acid.
- Functional Testing: Evaluate disintegration time and water absorption capacity.
- Safety and Impurity Testing: Measure pH, heavy metals, iron content, sodium chloride, sodium glycolate, and microbial contamination.
- Data Processing and Report Generation: Compile experimental data into a complete analytical report.
- Sample Amount: At least 5–10 g of SSG powder per test.
- Packaging: Sealed, dry, and protected from moisture and contamination.
- Sample Information: Batch number, manufacturer, production date, and relevant Safety Data Sheet (SDS).
- Shipping: Avoid high temperature and humidity; express or refrigerated shipping is recommended.
- Analytical report covering physical properties, chemical composition, functional performance, and safety/impurity results.
- Test data sheets and instrument raw data (provided on request).
- Recommendations and conclusions to support pharmaceutical R&D and quality control.
Sodium starch glycolate (SSG) is a highly efficient pharmaceutical excipient, primarily used as a superdisintegrant in solid dosage forms. SSG is synthesized from natural starches, such as corn, potato, or cassava, through chemical modification, acetylation, and sodium carboxymethylation. By introducing carboxymethyl or phosphate ester groups, starch molecules gain high water absorption and swelling capacity, significantly shortening the disintegration time of tablets and capsules. In pharmaceutical formulations, SSG not only accelerates drug dissolution but also improves tablet mechanical strength and uniformity.
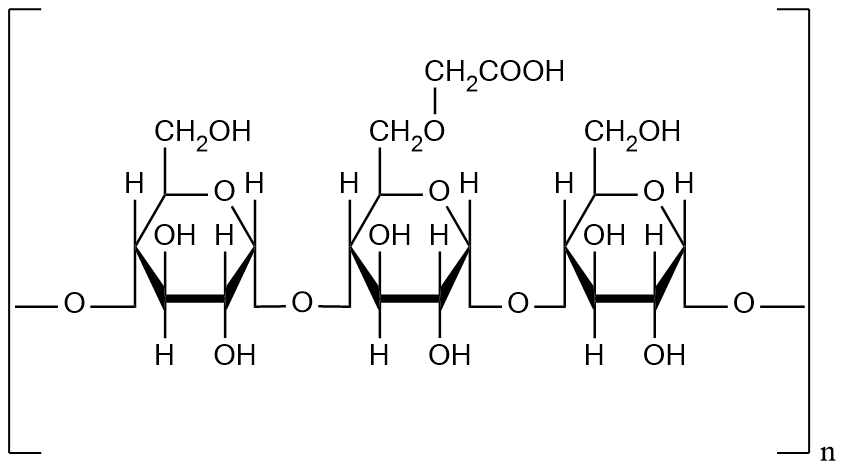
Figure 1. The Structure of Sodium Starch Glycolate
As a professional pharmaceutical excipient analysis service provider, MtoZ Biolabs provides a sodium starch glycolate analysis service that delivers a comprehensive evaluation of physical properties, chemical composition, and functional performance while fully adhering to international pharmacopeia standards to ensure excipient quality meets the requirements of pharmaceutical research and production. If you are interested in our service, please feel free to contact us at any time.
Services at MtoZ Biolabs
Our sodium starch glycolate analysis service covers a wide range of physical, chemical, functional, and safety tests. The specific analysis items are summarized below:
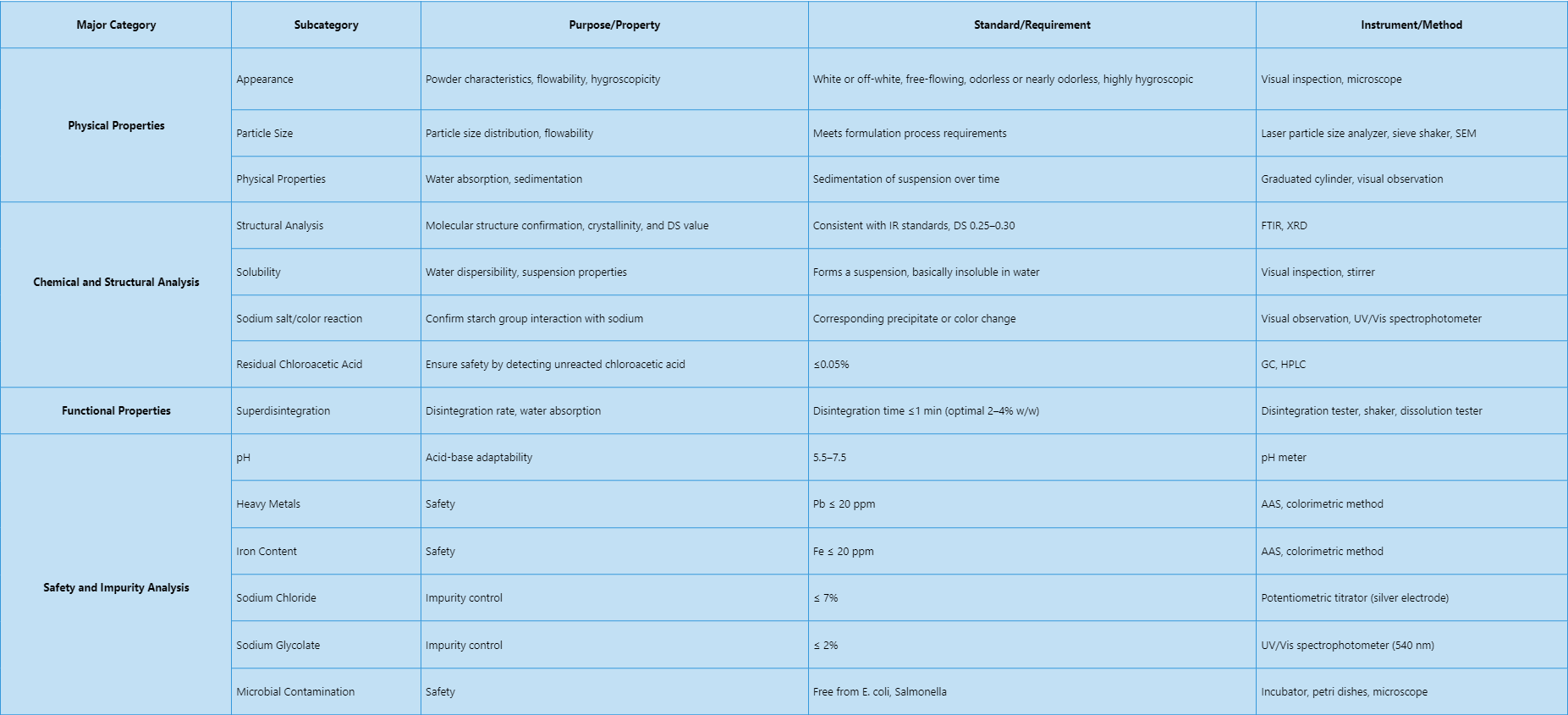
Analysis Workflow
Service Advantages
1. Comprehensive Coverage
MtoZ Biolabs’ sodium starch glycolate analysis service evaluates physical properties, chemical structure, functional performance, and safety, providing a thorough assessment from appearance and composition to disintegration behavior, supporting pharmaceutical development.
2. Pharmacopeia Compliance
All testing methods follow international pharmacopeia and industry standards, including USP and EP, ensuring results meet pharmaceutical excipient quality control requirements.
3. Advanced Instrumentation
Our sodium starch glycolate analysis service employs FTIR, XRD, laser particle size analyzers, UV/Vis spectrophotometers, AAS, GC, and HPLC to guarantee accurate and reliable data.
4. Rapid and Efficient Execution
Optimized workflows allow completion of routine and functional tests in a short time, supporting timely technical assistance for R&D and production.
5. Safety and Compliance Assurance
Testing for heavy metals, microbial contamination, residual chloroacetic acid, sodium chloride, and sodium glycolate ensures SSG excipients meet pharmaceutical safety requirements.
Applications
✔️Solid Dosage Form Development: Evaluate superdisintegrant performance in tablets and capsules.
✔️Pharmaceutical Quality Control: Pre-use testing of excipients before raw material release.
✔️Process Optimization: Assess SSG performance under various formulations and manufacturing conditions.
✔️Novel Excipient Development: Validate performance of modified starches or alternative starch sources.
FAQs
Q1: What Is the Standard for SSG Disintegration Testing?
A1: For formulations containing 2–8% SSG, 2–4% typically achieves disintegration within 1 minute per pharmacopeia guidelines.
Q2: Why Test for Residual Chloroacetic Acid?
A2: Chloroacetic acid may remain as a byproduct from SSG synthesis and must be controlled at ≤0.05% for pharmaceutical safety.
Q3: How Long Does the Sodium Starch Glycolate Analysis Service Take?
A3: Typically 7–10 business days, depending on the combination of testing items.
Sample Submission Suggestions
Deliverables
MtoZ Biolabs provides a comprehensive, professional sodium starch glycolate analysis service, supporting excipient quality assurance and formulation development. Whether for R&D stage excipient screening or production quality control, we deliver reliable data to ensure pharmaceutical safety, quality, and compliance.
Send your SSG samples to MtoZ Biolabs and receive a professional, authoritative analytical report to ensure your excipients meet high-quality pharmaceutical standards.
How to order?







