Single Particle Analysis (SPA) Service
Single Particle Analysis (SPA) Service is a high-resolution 3D structural analysis service based on the Cryo-Electron Microscopy (Cryo-EM) platform. It enables the three-dimensional reconstruction of individual biological macromolecular particles without the need for crystallization. This service is widely applied in key areas of life science and drug discovery, including molecular mechanism studies, antibody-binding mode analysis, and novel drug target validation.
Single Particle Analysis (SPA) is a 3D reconstruction technique that eliminates the requirement for crystallization and relies on cryo-EM data. It is widely used to resolve the high-resolution structures of biological macromolecules, such as protein complexes, viral particles, and ribosomes. The method involves automatic identification, image alignment, classification averaging, and 3D reconstruction of thousands to millions of 2D projection images of individual particles embedded in vitreous ice. The final outcome is an electron density map of the target molecule, which can then be used to build an atomic model.
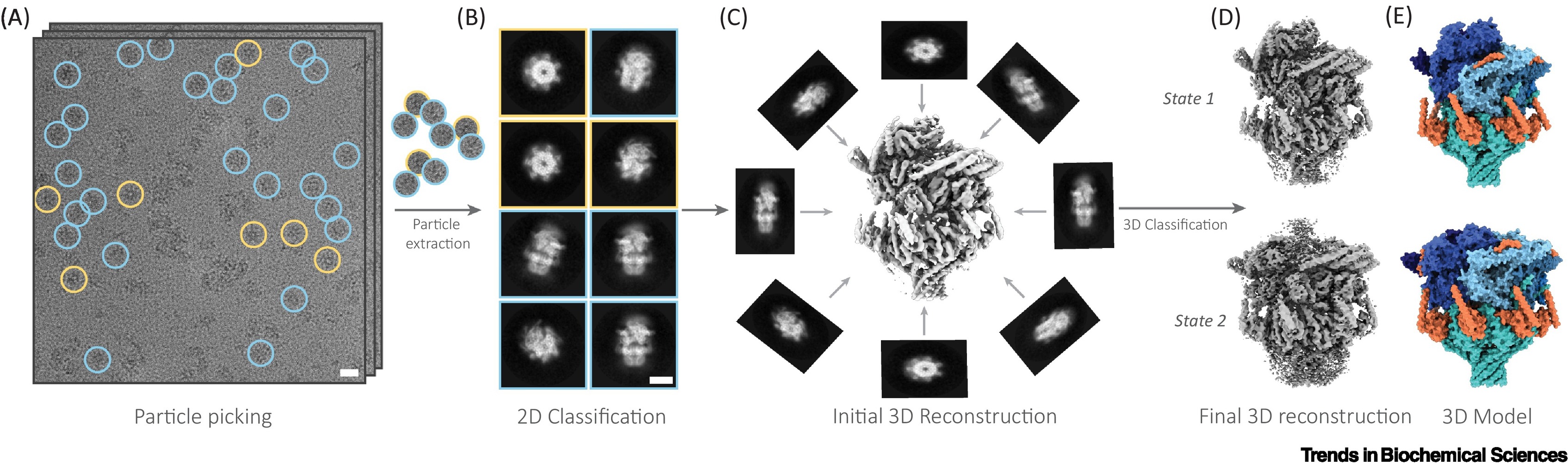
Anton L. et al. Trends in Biochemical Sciences. 2022.
SPA is applicable to most water-soluble or membrane-associated protein complexes, particularly those with high conformational flexibility, significant heterogeneity, or poor crystallizability. It provides near-atomic resolution structural information without disrupting the molecule’s native conformation, making it one of the core tools in modern structural biology, drug target elucidation, and mechanism-based research.
Leveraging an advanced cryo-electron microscopy platform, MtoZ Biolabs offers Single Particle Analysis (SPA) Service for 3D reconstruction of non-crystalline macromolecular complexes. This service enables researchers to obtain high-precision structural data while preserving the near-native state of the sample, supporting in-depth functional analysis and structure-guided research and development.
Analysis Workflow
Single Particle Analysis (SPA) Service at MtoZ Biolabs covers the complete process from sample preparation to structural modeling:
1. Sample Preparation and Grid Freezing
Evaluate sample quality, prepare cryo-EM grids, and perform plunge-freezing to vitrify the specimen.
2. Cryo-EM Imaging
Acquire a large dataset of single-particle images using cryo-electron microscopy.
3. Image Processing and 2D/3D Analysis
Apply motion correction, particle picking, 2D classification, and 3D reconstruction.
4. 3D Structure Refinement and Reporting
Deliver high-resolution electron density maps, resolution validation, and optionally, atomic model fitting reports.
Service Advantages
1. No Crystallization Required: SPA bypasses the need for crystal growth, making it ideal for non-crystalline proteins, membrane proteins, and large complexes—overcoming the major limitation of traditional X-ray crystallography.
2. Preserves Native Conformation: Samples are vitrified in vitreous ice, retaining their near-native state in solution and avoiding conformational shifts associated with crystallization.
3. Supports Conformational Heterogeneity:SPA enables classification and reconstruction of particles with multiple conformations, helping to reveal dynamic structural mechanisms.
4. Suitable for Very Large Complexes: While SPA has a lower molecular weight limit, it has no upper limit, making it ideal for structural analysis of large assemblies such as viruses, ribosomes, and multi-subunit enzyme complexes.
Applications
Single Particle Analysis (SPA) Service is broadly applicable in structural biology and especially suited for:
Viral Particles and Capsid Proteins
Elucidating assembly mechanisms and antibody-binding epitopes.
Membrane and Channel Proteins
Revealing conformational transitions in transmembrane domains and ligand-binding characteristics.
Large Protein Complexes
Including ribosomes, ribonucleoproteins, and transcriptional assemblies.
Conformationally Dynamic Systems
Structural characterization of polymorphic or transient molecular complexes.
Drug Target Validation
Defining binding mechanisms and structural bases of interaction with small molecules or antibodies.
Molecular Mechanism Studies
Integrating functional data with structural insights to understand critical biological processes.
FAQ
Q. Is SPA Suitable for All Types of Proteins?
SPA is best suited for large (>150 kDa), structurally stable, and monodisperse proteins. For smaller or highly flexible targets, we recommend pre-assessing sample purity and homogeneity.
Q. Is SPA the Same As Cryo-EM?
Not exactly. Cryo-EM refers to the experimental platform, whereas SPA is a computational method within cryo-EM used specifically for 3D reconstruction of single particles.
Case Study
This study utilized SPA to resolve the high-resolution 3D structure of the native Dystrophin-Glycoprotein Complex (DGC) from rabbit skeletal muscle. SPA reconstruction revealed the precise conformational relationships among multiple DGC components, including β-, γ-, δ-sarcoglycans, dystrophin, and α-dystroglycan. The analysis showed that these glycoproteins form a stable extracellular scaffold via β-helix architecture and connect the sarcolemma to the actin cytoskeleton, offering valuable insights into the structural basis of muscle integrity and dystrophinopathies.
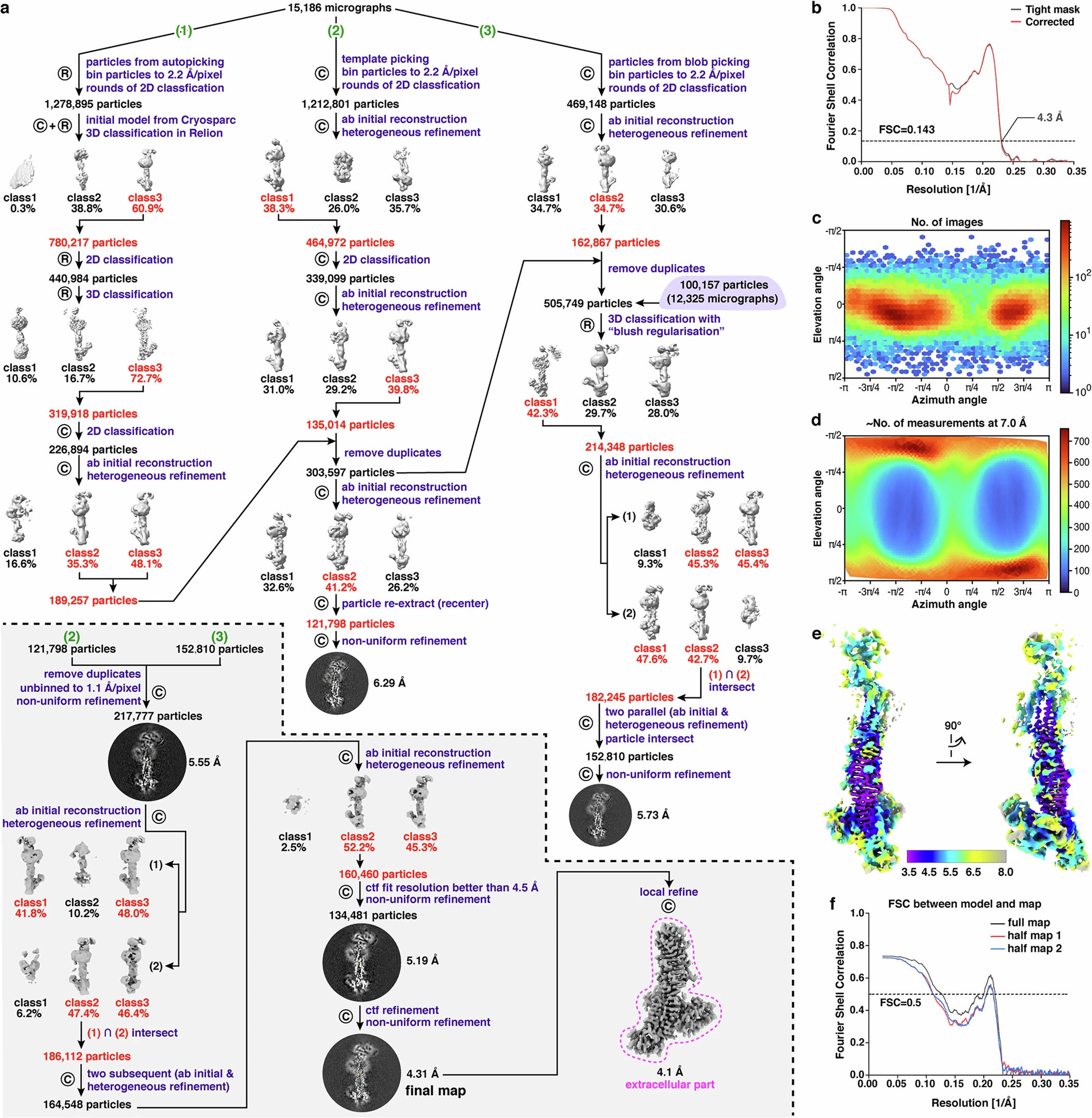
Liu S. et al. Nature. 2025.
How to order?







