Recombinant Collagen C-terminal Sequence Analysis Service
Recombinant Collagen C-terminal Sequence Analysis Service is a specialized service designed to precisely analyze the C-terminal amino acid sequence of recombinant collagen. It aims to validate the structural integrity, post-translational modifications (such as glycosylation, phosphorylation, etc.), and functionality of recombinant collagen.
Recombinant Collagen is collagen produced through genetic engineering techniques in host cells and is widely used in fields such as biomedicine, tissue engineering, skincare, and wound repair. During the production of recombinant collagen, ensuring the accuracy and integrity of the C-terminus is crucial. The C-terminal sequence not only affects the stability and function of the protein but also directly participates in biological processes such as interactions with other molecules and degradation mechanisms.
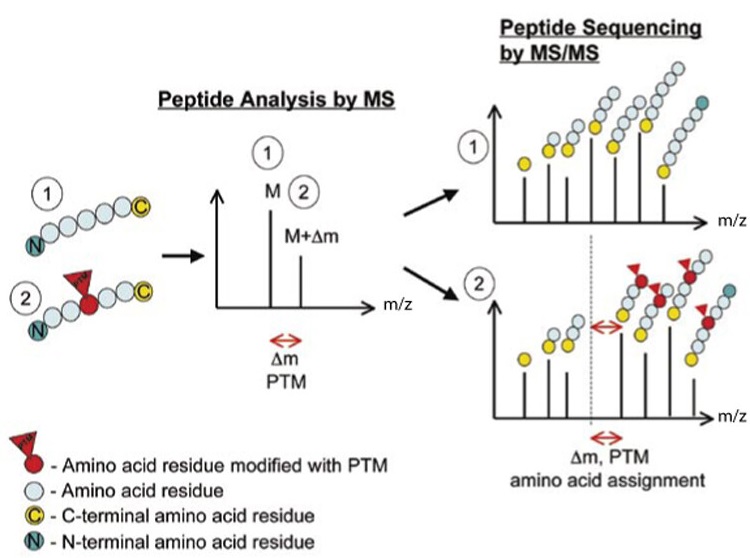
Larsen MR. et al. BioTechniques. 2018.
C-terminal Sequence Analysis is an important technique for analyzing the C-terminal amino acid sequence of proteins. It can be used to verify the correctness, structure, functionality, and potential post-translational modifications of recombinant collagen. Currently, C-terminal sequence analysis is primarily based on mass spectrometry. This method involves first selecting two different proteases to digest the target protein, producing two distinct C-terminal peptide fragments. These fragments are then analyzed using mass spectrometry. Finally, the two C-terminal sequences are combined to provide the complete sequence information of the C-terminal peptide.
Leveraging advanced mass spectrometry platforms, MtoZ Biolabs offers Recombinant Collagen C-terminal Sequence Analysis Service accurately deciphering the amino acid sequence of the C-terminal region and can reveal post-translational modifications that may impact collagen function. This service is applicable for quality control, functional validation, and optimization of recombinant collagen, ensuring its efficacy and stability in biomedicine, tissue engineering, and vaccine development.
Analysis Workflow
The main analysis workflow of Recombinant Collagen C-terminal Sequence Analysis Service is as follows:
1. Enzymatic Digestion and Sample Preparation
Digest recombinant collagen with two different proteases to produce small peptide fragments suitable for analysis.
2. C-terminal Sequence Analysis
Use ultra-high-resolution mass spectrometry to analyze the two enzymatic digestion products separately.
3. Data Analysis and Comparison
Utilize specialized software to analyze the mass spectrometry data, combine the results to confirm the C-terminal sequence, and compare it with the theoretical sequence.
4. Report Delivery
Provide a detailed C-terminal sequence report.
Service Advantages
1. Advanced Analysis Platform: MtoZ Biolabs established an advanced Recombinant Collagen C-terminal Sequence Analysis platform, guaranteeing reliable, fast, and highly accurate analysis service.
2. One-Time-Charge: Our pricing is transparent, no hidden fees or additional costs.
3. High-Data-Quality: Deep data coverage with strict data quality control. AI-powered bioinformatics platform integrates all Recombinant Collagen C-terminal Sequence Analysis data, providing clients with a comprehensive data report.
4. Customized Service: Based on the specific needs of different clients, MtoZ Biolabs offers tailored analytical solutions to help clients address specific structural issues.
Sample Submission Suggestions
MtoZ Biolabs accepts a variety of sample types, including purified recombinant collagen, lyophilized powders, and more. Samples should be shipped at low temperatures and protected from repeated freeze-thaw cycles.
If your sample conditions are special or if you're unsure how to prepare your samples, please feel free to contact the MtoZ Biolabs technical team. We will provide personalized sample submission guidelines and pretreatment support based on your needs.
Applications
Application examples of Recombinant Collagen C-terminal Sequence Analysis Service:
Recombinant Collagen Quality Control: Ensure that the C-terminal sequence matches the designed sequence and verify the accuracy of recombinant collagen.
Vaccine Development and Immunology Research: Ensure that the C-terminal structure of collagen is suitable for use as vaccine material, promoting immune responses.
Post-translational Modifications (PTMs) Analysis: Identify and quantify post-translational modifications in the C-terminal region, ensuring the optimization of collagen functionality.
Deliverables
1. Experimental Steps
2. Relevant Mass Spectrometry Parameters
3. Mass Spectrometry Images
4. Raw Data
5. Recombinant Collagen C-terminal Sequence Information
How to order?







