Protein PTMs Analysis Services
Protein PTMs analysis services focus on identifying and quantifying various types of post-translational modifications (PTMs) in proteins. After DNA undergoes transcription and translation to form nascent proteins, these proteins can be modified within cellular organelles such as the Golgi apparatus and endoplasmic reticulum, as well as in the cytoplasm, through enzymatic addition of specific modifying molecules to particular amino acid residues. These modifications vary widely in type and molecular weight, ranging from small modifications like methylation to large-scale modifications such as N-glycosylation, which can reach several thousand Daltons. These modifications endow proteins with diverse functionalities and enable the regulation of protein activity through biologically controlled modification and demodification processes, thereby facilitating various biological activities. By leveraging protein PTMs analysis technology, it is possible to achieve high-throughput qualitative and quantitative characterization of protein modifications within samples. This analysis provides deeper insights into biological mechanisms based on modification profiling results.
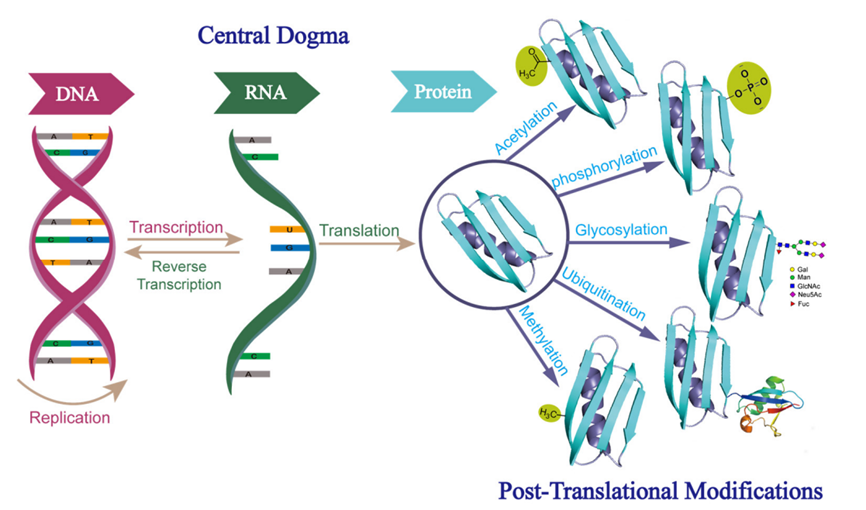
Chen, B Q. et al. Molecules, 2022.
Figure 1. Diverse Post-Translational Modification of Proteins.
Services at MtoZ Biolabs
Leveraging cutting-edge modification enrichment and high-resolution mass spectrometry acquisition technologies combined with bioinformatics analysis, MtoZ Biolabs has established a comprehensive protein PTMs analysis services platform. This platform includes phosphorylation, N-glycosylation, O-glycosylation, O-GlcNAc glycosylation, ubiquitination, SUMOylation, lactylation, and other PTM analysis services, providing qualitative and quantitative results for protein modifications in various samples. Our services cover the following key areas:
1. Phosphoproteomics Analysis Service
Protein phosphorylation is a critical mechanism in cellular regulation and signal transduction. It is estimated that approximately one-third of eukaryotic proteins undergo phosphorylation, primarily occurring on serine (S), threonine (T), and tyrosine (Y) residues. These phosphorylation modifications can alter protein conformation, stability, activity, subcellular localization, or protein-protein interactions. Phosphoproteomics is the large-scale analysis of phosphorylation sites, which are often found in low-abundance proteins. Phosphopeptide enrichment is a prerequisite for phosphoproteomics, and common enrichment methods include TiO₂ enrichment, anti-phosphotyrosine antibody enrichment, immobilized metal affinity chromatography (IMAC), chemical modification, and strong cation exchange chromatography (SCX). MtoZ Biolabs employs a stepwise TiO₂ enrichment strategy to effectively enhance phosphopeptide enrichment efficiency. After protein extraction and enzymatic digestion into peptides, phosphopeptides are enriched and analyzed via LC-MS/MS, ensuring high-throughput and accurate phosphoproteomics detection. By integrating bioinformatics analysis, we provide an in-depth exploration of biological questions related to phosphorylation modifications.
2. Glycoproteomics Analysis Service
Glycoproteomics is an important branch of PTM proteomics, focusing on identifying protein glycosylation modifications in cells, tissues, bodily fluids, or other sample types. Mass spectrometry-based glycoproteomics enables comprehensive analysis of glycan modifications, providing insights into glycan types, glycosylation sites, and abundance differences between groups. This approach has significantly advanced glycosylation research in recent years.
3. Lactylation Proteomics Analysis Service
Lactyl-proteomics is a component of post-translational modification (PTM) proteomics. Lactylation refers to the covalent attachment of lactate (or its derivatives) to lysine residues in proteins, forming lactyl modifications. These modifications serve diverse functions, potentially directly regulating the activity of enzymes involved in glycolysis and lactate production, thereby influencing cellular energy metabolism. Additionally, lactylation may act as a novel signaling mechanism, participating in intracellular and extracellular signal transduction processes. It can also impact transcription factor activity, thereby modulating gene expression. In recent years, multiple studies have demonstrated that lactylation is associated with cellular differentiation, tumorigenesis, and the development of various diseases.
MtoZ Biolabs provides DARTS, and drug target discovery services, employing Thermo Scientific’s TMT labeling reagents, Orbitrap, and Astral series mass spectrometers, combined with Nano-LC systems, ensuring high-precision and high-sensitivity data acquisition for comprehensive PTM characterization.
4. Additional PTM Analysis Services
Beyond the ubiquitination, SUMOylation, and O-GlcNAc glycosylation PTM proteomics services, MtoZ Biolabs has extensive project experience and offers high-specificity, high-sensitivity detection solutions tailored to various PTM studies.
Service Advantages
1. Advanced Analytical Platform
MtoZ Biolabs has established an advanced protein PTMs analysis services platform, ensuring high reliability, rapid turnaround, and high-precision results.
2. Transparent Pricing
Our pricing structure is fully transparent, with no hidden or additional fees.
3. High-Quality Data
The data coverage is extensive, and strict quality control measures are implemented. The bioinformatics platform integrates all post-translational modification (PTM) analysis data, providing clients with comprehensive data quality control, differential analysis, and functional analysis reports.
4. Customized Research Solutions
MtoZ Biolabs offers tailored services to address specific research questions and experimental requirements.
Sample Submission Suggestions
1. Sample Types
We accept cell and tissue samples. Solid samples can be transported using ice packs. For liquid samples, they can be vacuum-dried or freeze-dried before being transported with ice packs, or alternatively, shipped using dry ice. We ensure proper sample preservation to maintain protein integrity and achieve accurate quantification results.
2. Sample Amount
A minimum of three biological replicates is recommended.
Note: If you have special requirements or need assistance with sample preparation, please contact us.
Applications
1. PTM Profiling of Biological Samples
Protein PTMs analysis services can establish a comprehensive protein modification profile of samples, enabling a deeper understanding of higher-dimensional proteomics.
2. Biomarker Discovery
By comparing modified proteins between disease and healthy groups using PTM analysis techniques, differential modification proteins and biomarkers can be identified.
3. Modification Dynamics Study
Protein PTMs analysis services can be utilized to study modification level changes in different biological processes, providing insights into the role of modifications in developmental mechanisms.
4. Multi-Omics Analysis
Combining PTM analysis with transcriptomics, metabolomics, proteomics, and modification studies allows for a more comprehensive characterization of biological systems.
Case Study
1. Investigating the Molecular Mechanism of EMT in Hepatocellular Carcinoma Using TMT-Based Glycoproteomics Analysis
Epithelial-mesenchymal transition (EMT) has been recognized as a critical step toward high invasiveness and metastasis in various cancers, including hepatocellular carcinoma (HCC). However, the mechanisms promoting EMT remain unclear. This study employed a TMT-based glycoproteomics approach to systematically investigate the dynamic changes in specific glycosylation during the EMT process induced by HGF/TGF-β1 in three HCC cell lines. Additionally, multiple molecular biology techniques were used to further confirm the potential roles of EMT-associated glycoproteins and specific glycan structures. The study revealed that HGF or TGF-β1 treatment of HCC cells upregulated the glycosyltransferase FUT8, leading to increased core fucosylation, particularly on folate receptor α (FOLR1). This modification enhanced folate uptake and promoted EMT. Silencing FUT8 effectively blocked this process, demonstrating that FUT8 is a key driver of EMT in HCC cells.
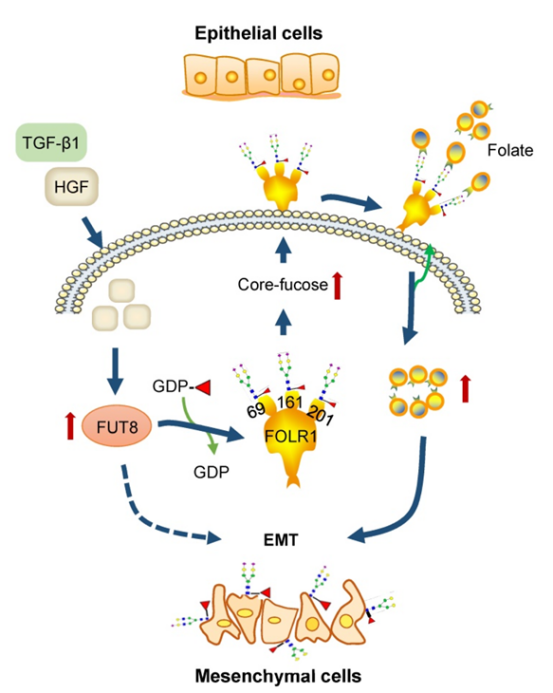
Li, J. et al. Theranostics, 2021.
Figure 2. Glycobiological Mechanism of EMT in Hepatocellular Carcinoma.
Deliverables
1. Comprehensive Experimental Details
2. Materials, Instruments, and Methods
3. Total Ion Chromatogram & Quality Control Assessment
4. Data Analysis, Preprocessing, and Estimation
5. Bioinformatics Analysis
6. Raw Data Files
MtoZ Biolabs, an integrated Chromatography and Mass Spectrometry (MS) Services Provider, provides advanced proteomics, metabolomics, and biopharmaceutical analysis services to researchers in biochemistry, biotechnology, and biopharmaceutical fields. Our ultimate aim is to provide more rapid, high-throughput, and cost-effective analysis, with exceptional data quality and minimal sample consumption. Free project evaluation, welcome to learn more details!
MtoZ Biolabs, an integrated chromatography and mass spectrometry (MS) services provider.
Related Services
Phosphoproteomics Analysis Service
How to order?







