Protein PTMs Analysis Service
Protein post translational modifications (PTMs) are a central source of protein diversity and functional complexity, playing critical roles in cellular signaling, protein-protein interactions, and disease mechanisms. Protein PTMs Analysis Service utilizes high-sensitivity mass spectrometry combined with nano-liquid chromatography systems to precisely detect specific modified peptide fragments, bioinformatics tools to analyze mass spectrometry data, enabling the localization of modification sites, identification of modification types, and quantification of dynamic changes in modifications. MtoZ Biolabs offers Protein PTMs Analysis Service covering a wide range of modifications, including phosphorylation, ubiquitination, glycosylation, acetylation, and methylation. Customizable solutions are available to meet the needs of complex research projects, such as studying phosphorylation signaling pathways, investigating the role of glycosylation in diseases, or identifying ubiquitination-mediated degradation mechanisms.
Analysis Workflow
Bagwan N. et al. Scientific Reports. 2021.
Sample Preparation
Extract and digest proteins, ensure high sample purity.
Enrichment of Specific Modifications
Enrich modified peptides using targeted techniques, such as TiO₂ column enrichment for phosphorylated peptides.
Mass Spectrometry Detection
Obtain high-resolution data using the LC-MS/MS platform, ensuring sensitive and accurate detection of modified peptides.
Data Analysis
Identify, localize, and quantify modifications using specialized software.
Report Generation
Deliver detailed results, including modification analysis and biological significance interpretation,.
Applications
Applications of protein PTMs analysis service including:
Studying key regulatory mechanisms in signaling pathways (e.g., phosphorylation, acetylation).
Exploring abnormal modification patterns in diseases, such as glycosylation changes associated with cancer.
Identifying novel biomarkers to support the development of diagnostic and therapeutic strategies.
Evaluating the impact of drugs on target protein modifications to assist in drug development.
Deliverables
1. Comprehensive Experimental Details
2. Materials, Instruments, and Methods
3. Total Ion Chromatogram & Quality Control Assessment (project-dependent)
4. Data Analysis, Preprocessing, and Estimation (project-dependent)
5. Bioinformatics Analysis
6. Raw Data Files
FAQ
Q. How to Simultaneously Analyze Multiple Modifications on the Same Protein (e.g., Coexistence of Phosphorylation and Acetylation)?
The coexistence analysis of multiple modifications can reveal the synergistic effects and regulatory mechanisms between protein modifications, but the analysis is difficult. The following are specific solutions:
1. Sample Preparation and Enrichment
Step Enrichment: Use IMAC to enrich phosphorylated peptides first, followed by antibody-based capture of acetylated peptides. Step enrichment minimizes data loss caused by competition between modifications.
Combined Enrichment: Employ strategies capable of capturing multiple modifications simultaneously, such as strong cation exchange (SCX) combined with secondary separation techniques.
2. Mass Spectrometry Detection
Utilize multiple fragmentation modes (e.g., HCD combined with ETD) to comprehensively cover fragment features of modified peptides.
Apply DIA (Data-Independent Acquisition) for full-scan analysis, ensuring high coverage of modified peptides.
3. Bioinformatics Analysis
Use software that supports the identification of coexisting modifications (e.g., MaxQuant) and configure multiple variable modification parameters.
Employ a custom modification database to enhance the accuracy of modification identification.
By combining appropriate enrichment strategies, advanced mass spectrometry techniques, and precise bioinformatics tools, comprehensive analysis of multiple modifications on a single protein can be achieved.
Case Study
This study utilized protein PTMs analysis to identify a conserved lactylation at ALDOA-K147. Combining genetic code expansion technology, mass spectrometry analysis, and functional validation, researchers revealed that lactylation modifications significantly alter the protein’s structure and biological functions, including regulation of enzyme activity and protein-protein interactions.
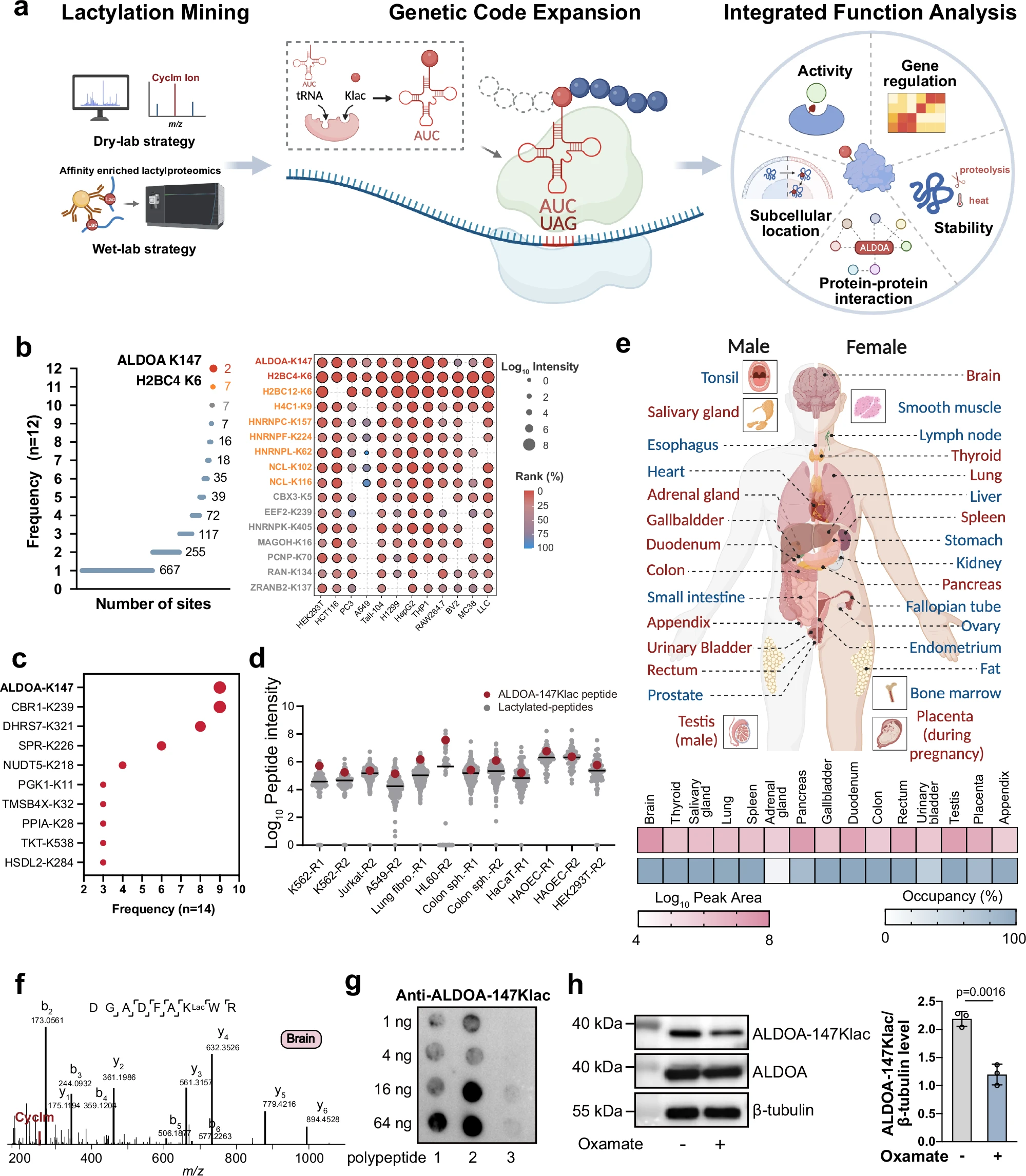
Shao C. et al. Nat Commun. 2025.
MtoZ Biolabs, an integrated Chromatography and Mass Spectrometry (MS) Services Provider, provides advanced proteomics, metabolomics, and biopharmaceutical analysis services to researchers in biochemistry, biotechnology, and biopharmaceutical fields. Our ultimate aim is to provide more rapid, high-throughput, and cost-effective analysis, with exceptional data quality and minimal sample consumption.
MtoZ Biolabs, an integrated chromatography and mass spectrometry (MS) services provider.
Related Services
PTM Sites Identification Service
PTM Quantitative Analysis Service
Histone Post-Translational Modification (PTM) Proteomics Service
How to order?







