Protein Corona-Mediated Nanomedicine Targeted Delivery Service
Nanoparticles (NPs) undergo interactions with serum proteins during systemic administration, which may result in the formation of protein corona (PC). Investigating the interplay between the chemical and biological properties of NPs enhances clinical translation efficiency. NPs with PC can be considered to exhibit a distinct biological characteristic, which engage with cells and barriers within biological systems to influence cellular uptake, cell fate, macrophage uptake, biodistribution, and targeting efficiency. The primary goal in developing NPs for drug delivery is to amplify drug concentration at targeted sites while minimizing its distribution in normal tissues. Recent studies indicate that the selection of different materials can affect the adsorption patterns of proteins on NP surfaces, and subsequently enhance drug administration effectiveness. Over recent years, two principal strategies, passive and active targeting drug delivery, have been evolved. Hence, it is vital to understand the biological characteristics of NP-protein complex and its influence on targeting behaviors to advance targeted drug delivery systems.
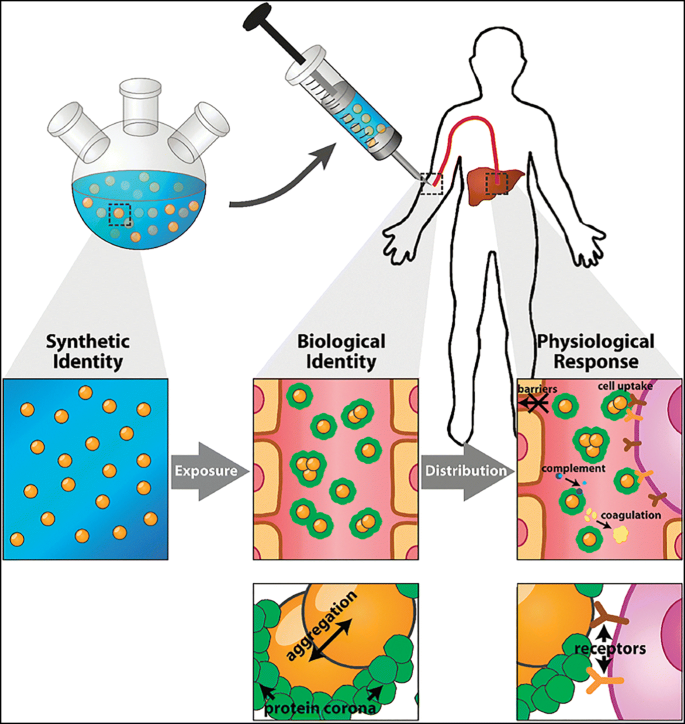
Figure 1. Systemic Delivery and Targeting of NPs Driven by PC [3]
PC formation and the exchange of proteins adsorbed under biological conditions evolve over time. The composition of PC is largely determined by the NPs' physicochemical properties (e.g., nanomaterials, size, charge, surface functional groups, shape) and exposing environments including immersion media components, temperature, pH, dynamic shear stress, and duration of interaction time. Numerous proteins firstly bind to NP surface initially and are subsequently replaced by higher affinity proteins, a process known as Vroman effect.
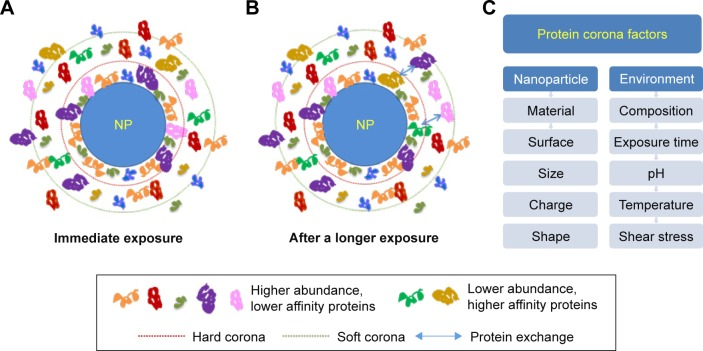
Figure 2. Factors Influencing the Formation of the Protein Corona [4]
1. Targeting Ligands for NP Therapy
Targeted drug delivery using protein ligands involves two prevalent strategies. The first utilizes natural proteins as targeting ligands, maintaining their inherent structure to simulate intrinsic protein-protein interactions. The second emphasizes novel design and engineering to create engineered biomolecules, particularly antibodies, for binding to target sites with high specificity and affinity.
(1) Natural Protein
Transferrin (Tf), an endogenous protein, transports iron throughout the body via the bloodstream and is a widely investigated natural protein ligand for NP drug targeting. The transferrin receptors (TfR) are overexpressed on various tumor cell membranes and are extensively present on the blood-brain barrier (BBB), which facilitates the targeting of NP drugs at persistent brain disorders. Tf remains the most extensively studied natural protein ligand for targeted delivery of NP drugs with other drugs less documented.
(2) Engineered Antibody
Engineered antibody offers high specificity and affinity for their respective antigens, showing significant potential for diseases requiring selective target. Nonetheless, potential immunogenicity of engineered antibody remains a major limitation for its broader application. Antibody Fc fragments can expedite the elimination of NP drugs by activating the mononuclear phagocyte system (MPS) and the complement system, in which PC plays a pivotal role. For example, trastuzumab, a commercially successful anti-HER2 antibody, has been employed as a targeted ligand for anti-tumor NP drugs, and studies indicated that a NP bound to two trastuzumabs can moderately improve targeting efficacy.
(3) Aptamer
Aptamer is oligonucleotide targeting specific receptors with high affinity. In theory, aptamer could enhance the solubility and permeability of NP drugs. However, their sensitivity to environmental stimuli due to their chemical nature means can be high because slight changes in the microenvironment could greatly impact targeting function of aptamer. Hence, in the presence of plasma, the in vivo half-life of nucleic acid aptamer is typically short due to high clearance and rapid nuclease-mediated degradation. Therefore, the influence of the PC on aptamers is noteworthy.
(4) Small Molecule (SM)
Various SM ligands are employed in targeted drug delivery, yet interactions between these ligands and PC are less studied. Research indicated that PC in breast cancer patients' plasma more impeded the efficiency of folate-conjugated NP carriers compared to that in healthy donors. Investigations into PC on functionalized NP drugs could be crucial for the future clinical translation of targeted delivery.
(5) Peptide
Peptide ligands offer effective solutions for targeted drug delivery due to their moderate size, high permeability, and low immunogenicity. Their diverse chemical properties are easy to manipulate, with many peptides already developed as targeting ligands. More studies are required to identify and analyze key plasma proteins IgM that determine the fate of NP drugs in vivo, which can help the preclinical design and precise application of targeted NP therapies.
2. Modulating the PC to Enhance Targeted Delivery
In NP-based targeted delivery, there are two primary strategies: passive and active targeting delivery. Passive targeting delivery leverages the leaky properties of tumor microvessels and the stealth capabilities of NPs to promote permeability and retention time, leading to greater tumor accumulation. In contrast, active targeting delivery capitalizes on the overexpression of specific surface ligands on tumor cells compared to normal cells, such as folate and epidermal growth factor receptors or NPs that possess functionalized surfaces, to facilitate precise homing and selective tumor cell uptake.
Given the critical role of PC in targeted drug delivery, improving targeted delivery involving PC has become a focus. Numerous strategies have been proposed to minimize the adsorption of undesirable plasma proteins to enhance the stealth capabilities of nanomedicines. Additionally, several studies have emphasized the proactive modulation of PC to exploit its benefits for more effective targeted delivery.
(1) Surface Modification of NPs
Over the years, polyethylene glycol (PEG)ylation has consistently been the most commonly method for improving the drug delivery of NPs. Typically, NPs with more hydrophobic surfaces tend to attract a higher abundance of opsonin proteins, hastening their clearance by the immune system (MPS). PEGylation furnishes a substantial hydrophilic shield around NPs, spatially preventing the adsorption/binding of neighboring proteins. Exposure of PEG chains in plasma results in the formation of a PC rich in ApoJ, which has been shown to effectively inhibit MPS uptake of nonspecific cells. By providing a stealth coating, PEGylation alleviates the aggregation, opsonization, and phagocytosis induced by PCs, significantly prolonging the circulation of NPs in the bloodstream. Besides PEG, numerous other amphiphilic polymers have been explored to achieve enhanced functionalities. PEGylation fails to modulate surface charge, and charged surfaces of NPs often lead to increased protein adsorption. Research has investigated copolymers composed of PEG and poly(allyl glycidyl ether) (PEG-b-AGE) to encapsulate RGD/Tf-modified NPs to enhance PEG chains. The AGE segment can reduce PC formation by modulating surface charge and demonstrates anti-biofouling properties. Additionally, neutral poly electrolytes containing cationic and anionic functional groups, known as zwitterionic polymers, are gaining attention for providing stealth coating for NPs.
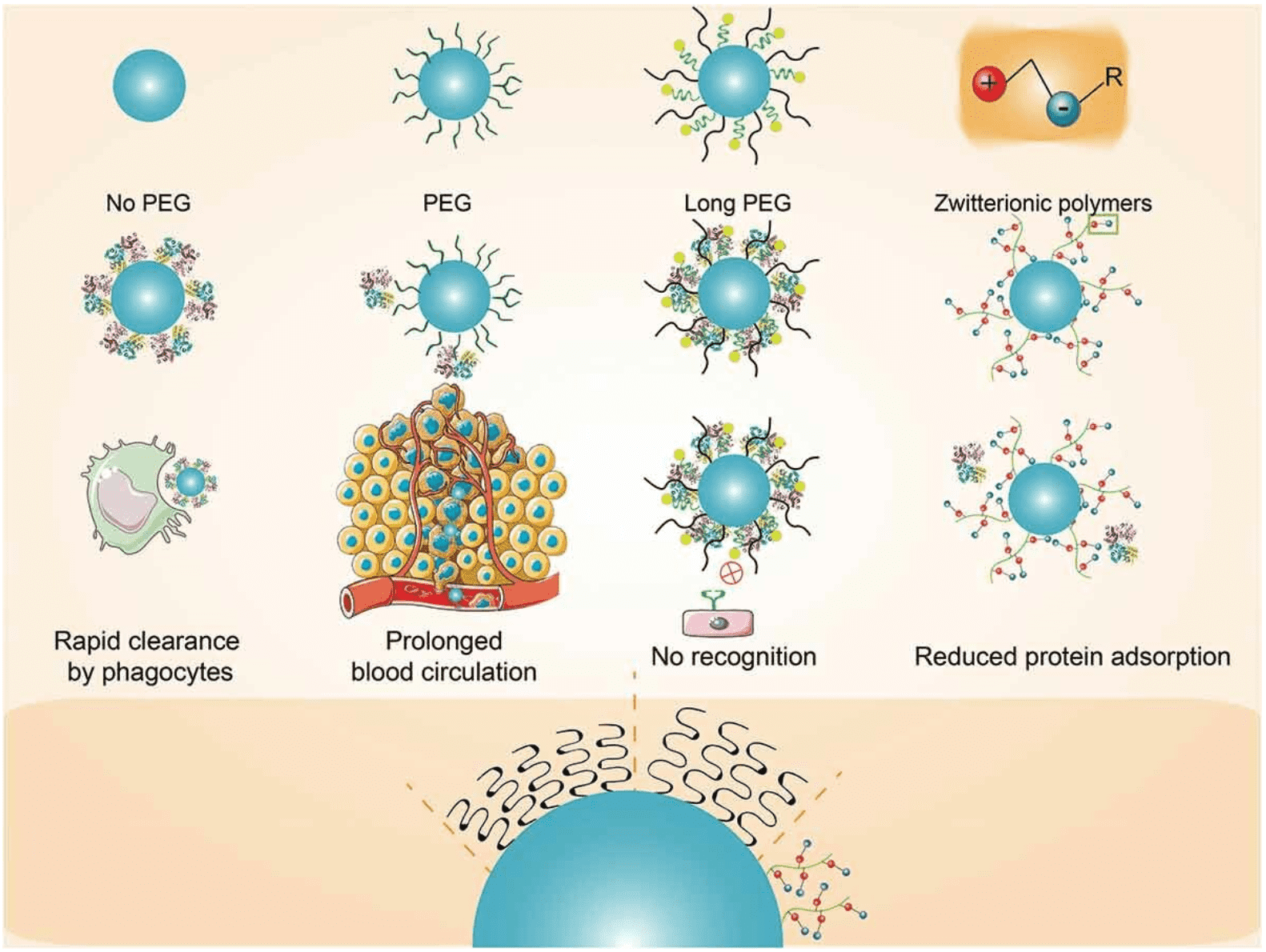
Figure 3. Modulating PC Formation to Enhance Targeting [5]
(2) Coating of NPs
By emulating the continually refined strategies throughout natural evolution, biomimetic membranes offer comprehensive stealth coatings for NPs and achieve excellent biocompatibility and prolonged circulation time. Several methods have been explored to provide biomimetic coatings for targeted NPs by utilizing cell membrane coatings and virus-like particles. Cell membrane cloaking is the most widely adopted technique for obtaining biomimetic stealth NPs and it offers intrinsic biological properties that result in enhanced immunocompatibility and reduced protein adsorption. Red blood cell (RBC) membranes are commonly utilized for NP encapsulation, as the absence of a cell nucleus facilitates the engineering of cell-to-carrier, and self-protective proteins such as CD47 on RBC membranes also impede the recognition by MPS. Virus represent another crucial resource for designing biomimetic coatings, as their fully evolved capsid structures enable them to disguise themselves from immune system clearance, providing a means to shield NPs from host immune system attacks. By mimicking viral infection stages, NPs effectively evade immune surveillance, traverse multiple barriers, enter cells, and release their payloads.
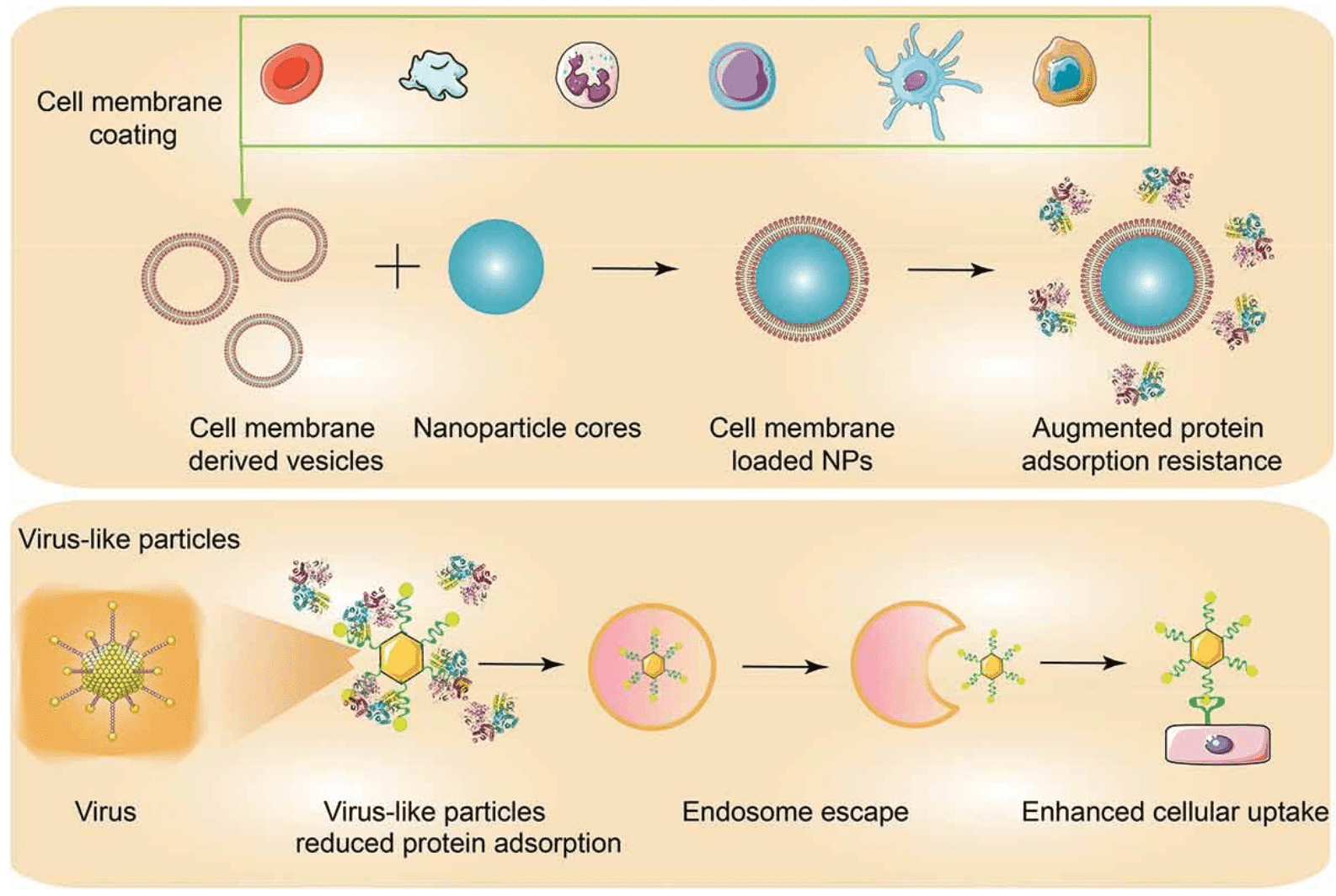
Figure 4. Coating of Nanoparticles to Enhance Targeting [5]
(3) Hidden Target
Numerous popular targeting drugs, such as Tf, FA, and RGD, have been found to exhibit affinity towards plasma proteins, particularly opsonin protein, resulting in an intensified PC that impedes targeted delivery. Hence, drugs with high affinity for target sites and specific interactions with plasma proteins represent potential alternative candidates. Rational molecular design emerges as a promising avenue for obtaining stealth targeting ligands. Notably, research has employed computer-aided design to develop a small peptide, D8, which attenuated natural IgM absorption while preserving CDX's targeting ability towards nAChRs. The development of D8 sheded light on the rational design of immunocompatible drug delivery systems by modulating PC composition.
(4) Protein Pre-Packaging
Pre-packaging NPs with specific proteins before administration stands as a primary strategy for manipulating PC. Pre-packaging proteins pre-formed PC in vitro. Furthermore, pre-formed PC can engage in further interactions with endogenous proteins post-administration. The simplest approach to this strategy involves "pre-adsorption", in which NPs are directly incubated with specific proteins, yielding a pre-formed PC. Albumin emerges as a favored inert protein, boasting outstanding biocompatibility and ready availability. Noteworthy, albumin modified with targeting ligands has become a common tool for pre-packaging NPs, resulting in a "ligand-albumin-nanoparticle" structure. However, it is imperative to recognize that in vitro pre-packaging with specific proteins does not ensure effective functionalization, as in vivo plasma proteins may interchange or scavenge the specific proteins, leading to the formation of more complex PCs.
(5) Protein Recruitment
Functionalized PCs can naturally formed through the active recruitment of specific proteins in vivo, augmenting targeted NP delivery. Considering that PCs might be influenced by NP formulation composition and particle surface charge, the diversity of lipid composition in NPs presents an opportunity to manipulate PCs on NP. Moreover, rational molecular design, particularly peptide design, facilitates accessibility to this recruitment strategy since peptide ligands are easy to manipulate and simulate medium-sized protein-protein interactions.
Consequently, the primary strategies for the targeted design of novel NPs encompass three steps. Firstly, the accumulation of active proteins can be further intensified to guide NPs to the desired sites. Secondly, identifying NP-specific features that enhance the recruitment of desired proteins and achieve appropriate PC formation with or without altering the native protein structure, to pursue the desired targeting response. Thirdly, considering the temporal impact of protein exchange on biological and therapeutic outcomes.
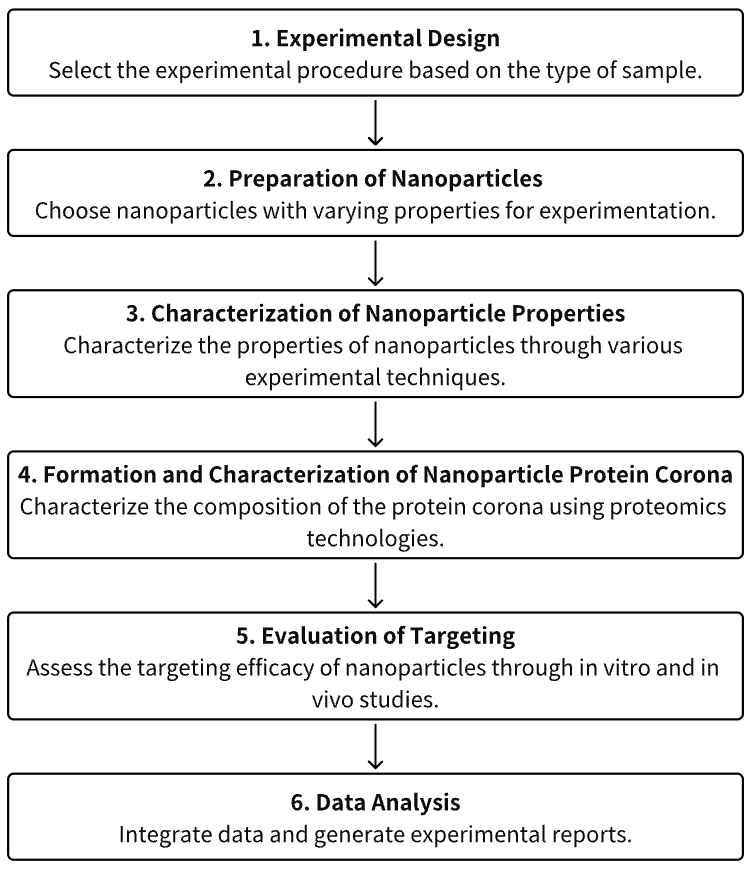
Analysis Workflow
1. Experimental Protocol Determination According to Experimental Requisites
2. Preparation and Optimization of Nanoparticles
3. Characterization of Nanoparticle Properties
4. Formation and Characterization of Nanoparticle Protein Corona
5. Assessment of Nanoparticle Targeted Therapeutic Efficacy Through in Vitro/in Vivo Evaluations
6. Data Analysis
Service Advantages
1. Full-Service Nanomedicine Development Including Preparation, Characterization and Evaluation
2. Highly Reliable and Precise Detection Methods
3. Comprehensive Bioinformatics Analysis
Example Results
1. Protein Corona Mediated Liposomal Drug Delivery for Bacterial Infection Management
Liposomes are a promising antibiotic delivery system widely investigated for treating life-threatening bacterial infections. However, the inevitable formation of PC on their surfaces significantly affects their in vivo performance. Enhancing our understanding for the impact of PC on liposomal behavior could greatly improve antimicrobial liposomal drug development. Research has highlighted the critical role of PC in the interactions between liposomes and bacteria. Negatively charged proteins adsorbed on cationic liposomes weakened electrostatic attraction enhanced liposomal binding to the bacteria. Anionic liposomes made of DSPG sLip, which accumulate complement deposits, show exceptional binding affinity for planktonic bacteria and biofilms, enhancing bacteria-targeted drug delivery. In both S. aureus-related osteomyelitis and pneumonia mice models, DSPG sLip has been demonstrated to be a highly promising antibiotic nanoparticle carrier for treating MRSA infections, indicating the benefits of lipid composition-based protein corona modulation in liposomal antibiotic delivery.
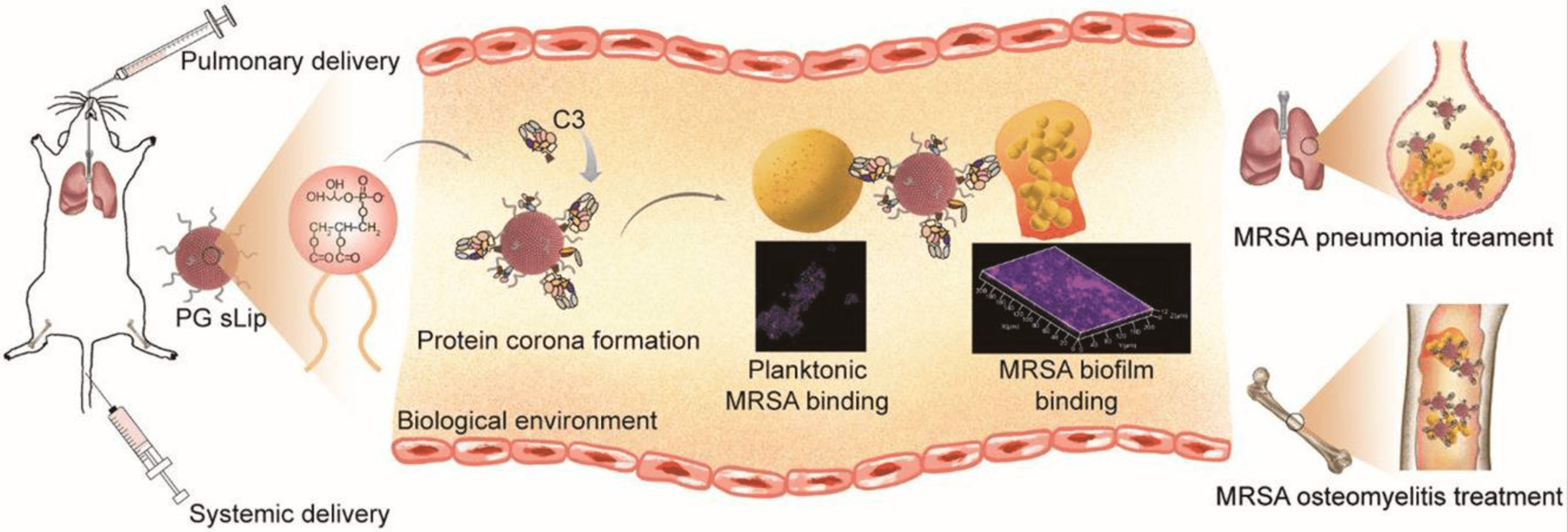
Figure 5. Liposomal Drug Delivery Mediated by Protein Corona for Bacterial Infection Management[6]
2. Brain-Targeted Drug Delivery by Manipulating Protein Corona Function
PC significantly hinders bench-to-bedside transition of targeted drug delivery systems, impacting the targeting yields and resulting in unfavorable biodistribution. Targeting mediated by PC, through precise manipulation of nanoparticle surface interactions with functional plasma proteins, offers new possibilities for specific drug delivery. Bio-inspired liposomes (SP-sLip) have been developed by modifying liposomal surfaces with non-toxic peptides derived from Aβ1-42, specifically interacting with the lipid-binding domains of exchangeable apolipoproteins. SP-sLip absorbed plasma apolipoproteins A1, E, and J, exposing their receptor-binding domains for effective brain-targeted delivery. Doxorubicin loaded SP-sLip (SP-sLip/DOX), showed signifificant enhancement of brain distribution and anti-brain cancer effect in comparison to doxorubicin loaded plain liposomes. SP-sLip maintained the functional properties of absorbed human plasma ApoE, and its corona-mediated targeting strategy was effective in SP modified PLGA NPs. This research could facilitate the clinical advancement of targeted drug delivery systems.
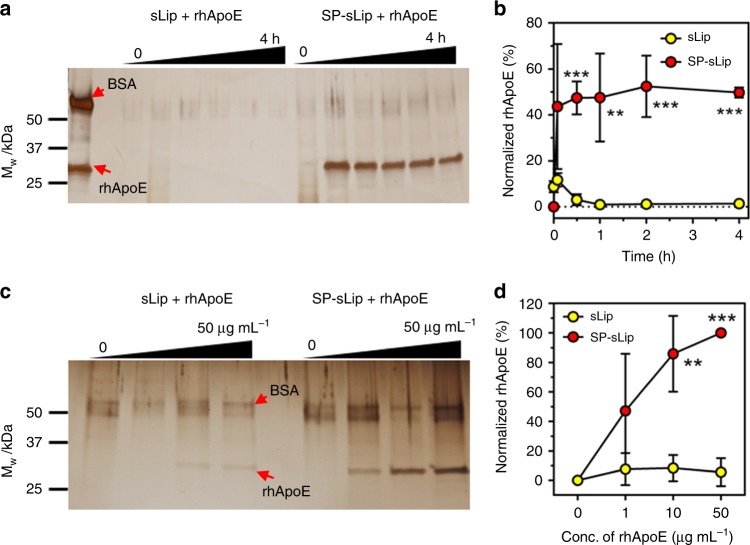
Figure 6. Binding Kinetics of Recombinant Human ApoE on Liposome Surfaces[7]
3. Cholesterol-Mediated Protein Corona Engraftment on DNA Nanostructures for Targeted Oligonucleotide Delivery for Liver Fibrosis Treatment
The PC, a layer of proteins formed on NP surfaces in vivo, significantly influences the fate of NP. Although precisely controlling protein adsorption on NPs remains challenging, exploiting the PC with ligands that have increased affinity for target tissue- and cell-homing proteins can enhance targeted drug delivery. A study has developed a trivalent cholesterol conjugation (Chol3-Td), enhancing interactions with serum lipoproteins and inducing a lipoprotein-associated PC in situ on DNA nanostructures in liver cells of healthy and myofibroblasts in fibrotic mice. Chol3-Td enhanced liver delivery of antisense oligonucleotides (ASO) targeting TGF-β1 mRNA, offering a treatment for liver fibrosis. The efficacy of ASO@Chol3-Td, compared to the clinically approved trivalent N-acetylgalactosamine (GalNAc3), underscored Chol3-Td's potential as a targeted delivery system for oligonucleotide therapeutics. This approach suggested that controlled seeding of the PC on nanomaterials can guide NPs to targeted regions effectively.
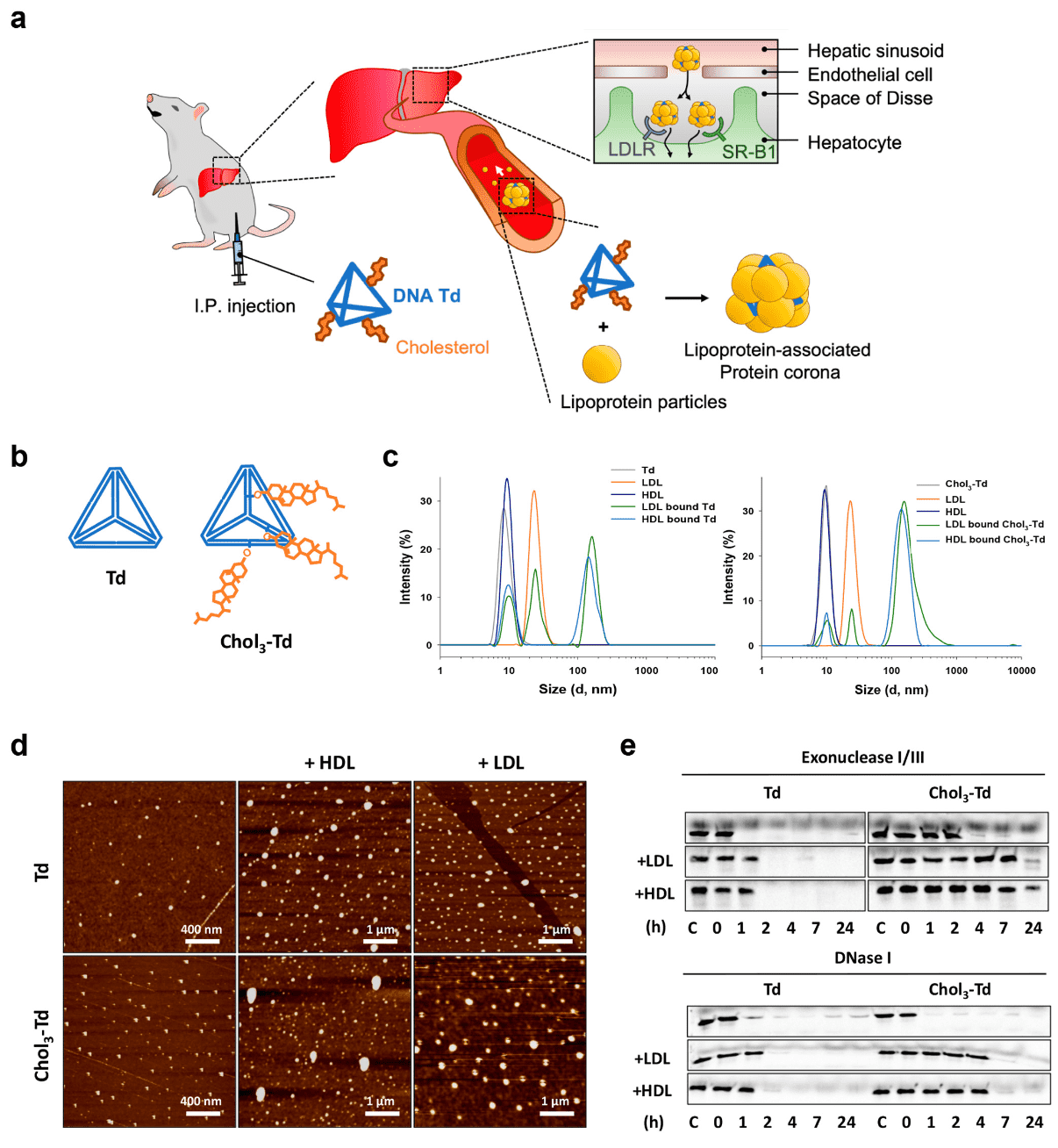
Figure 7. Cholesterol-Mediated Protein Corona Engraftment on DNA Nanostructures for Targeted Delivery in Liver Fibrosis[8]
Sample Submission Requirements
1. Minimization of Sample Impurities Contamination
Services at MtoZ Biolabs
1. Comprehensive Experimental Procedures Description
2. Pertinent Instrument Parameters
3. Original Experimental Data Set
4. Data Analysis Detailed Report Including Findings and Interpretations
Applications
1. Protein Corona Nanoparticle-Mediated Targeted Vaccine Delivery
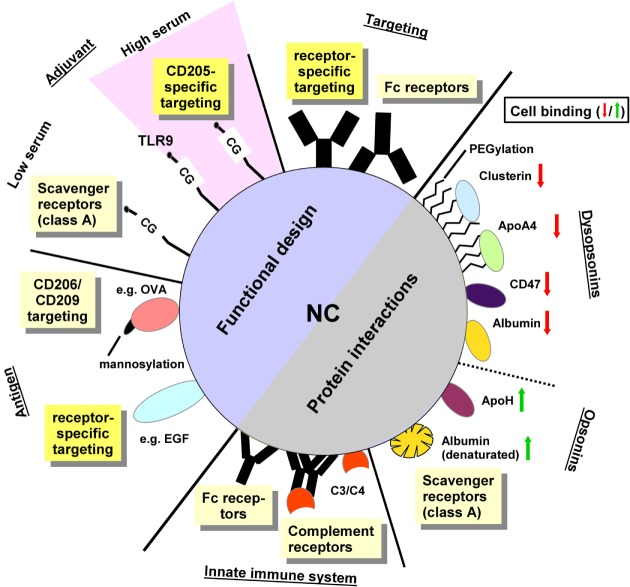
Figure 8. Protein Corona Nanoparticle-Mediated Targeted Vaccine Delivery[9]
FAQ
Q1: What are the effects of NP parameters on PC alterations?
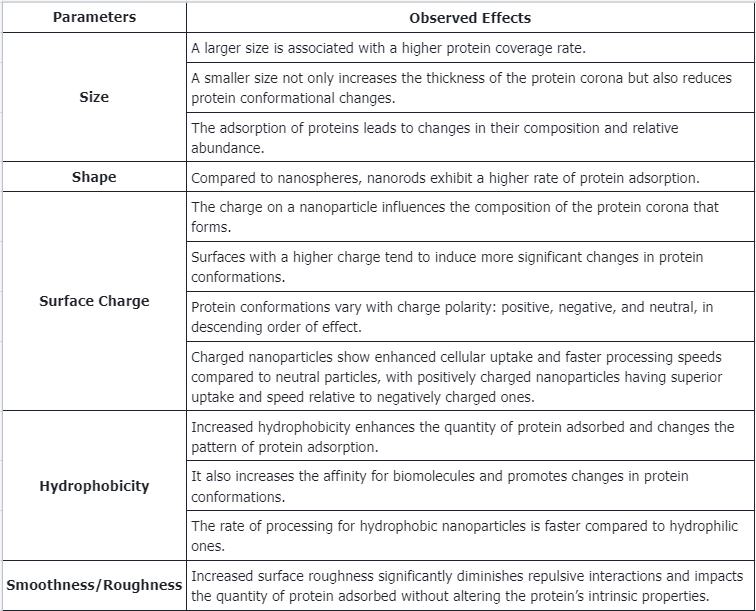
References
[1] Kim W, Ly NK, He Y, Li Y, Yuan Z, Yeo Y. Protein corona: Friend or foe? Co-opting serum proteins for nanoparticle delivery. Adv Drug Deliv Rev. 2023 Jan;192:114635. doi: 10.1016/j.addr.2022.114635. Epub 2022 Nov 26. PMID: 36503885; PMCID: PMC9812987.
[2] Liu Y, Wang J, Xiong Q, Hornburg D, Tao W, Farokhzad OC. Nano-Bio Interactions in Cancer: From Therapeutics Delivery to Early Detection. Acc Chem Res. 2021 Jan 19;54(2):291-301. doi: 10.1021/acs.accounts.0c00413. Epub 2020 Nov 12. PMID: 33180454.
[3] Chen D, Ganesh S, Wang W, Amiji M. Protein Corona-Enabled Systemic Delivery and Targeting of Nanoparticles. AAPS J. 2020 Jun 3;22(4):83. doi: 10.1208/s12248-020-00464-x. PMID: 32495039.
[4] Nguyen VH, Lee BJ. Protein corona: a new approach for nanomedicine design. Int J Nanomedicine. 2017 Apr 18;12:3137-3151. doi: 10.2147/IJN.S129300. PMID: 28458536; PMCID: PMC5402904.
[5] Jiang Z, Chu Y, Zhan C. Protein corona: challenges and opportunities for targeted delivery of nanomedicines. Expert Opin Drug Deliv. 2022 Jul;19(7):833-846. doi: 10.1080/17425247.2022.2093854. Epub 2022 Jun 27. PMID: 35738018.
[6] Shao Q, Ding T, Pan F, Li G, Shen S, Qian J, Zhan C, Wei X. Protein corona mediated liposomal drug delivery for bacterial infection management. Asian J Pharm Sci. 2022 Nov;17(6):855-866. doi: 10.1016/j.ajps.2022.10.003. Epub 2022 Nov 5. PMID: 36600900; PMCID: PMC9800951.
[7] Zhang Z, Guan J, Jiang Z, Yang Y, Liu J, Hua W, Mao Y, Li C, Lu W, Qian J, Zhan C. Brain-targeted drug delivery by manipulating protein corona functions. Nat Commun. 2019 Aug 8;10(1):3561. doi: 10.1038/s41467-019-11593-z. PMID: 31395892; PMCID: PMC6687821.
[8] Kim KR, Kim J, Back JH, Lee JE, Ahn DR. Cholesterol-Mediated Seeding of Protein Corona on DNA Nanostructures for Targeted Delivery of Oligonucleotide Therapeutics to Treat Liver Fibrosis. ACS Nano. 2022 May 24;16(5):7331-7343. doi: 10.1021/acsnano.1c08508. Epub 2022 May 2. PMID: 35500062.
[9] Bros M, Nuhn L, Simon J, Moll L, Mailänder V, Landfester K, Grabbe S. The Protein Corona as a Confounding Variable of Nanoparticle-Mediated Targeted Vaccine Delivery. Front Immunol. 2018 Aug 2;9:1760. doi: 10.3389/fimmu.2018.01760. PMID: 30116246; PMCID: PMC6082927.
MtoZ Biolabs, an integrated chromatography and mass spectrometry (MS) services provider.
Related Services
Protein Corona Characterization Service
MS-Based Protein Corona Characterization Service | Nanoparticles
How to order?







