Principles of iTRAQ-Based Quantitative Proteomics and Reporter Ion Analysis
-
Reporter Group, generating ions in the 113-121 Da range (8-plex system) that provide quantitative signals.
-
Balance Group, which compensates for mass differences and ensures identical total tag mass (~305 Da).
-
Peptide Reactive Group, typically an NHS ester, enabling covalent labeling of peptide N-termini and lysine residues.
-
Removal of background noise
-
Normalization across reporter channels
-
Aggregation of peptide-level data to protein-level abundance
-
Multiplexing capability: enables simultaneous quantification of up to eight samples in a single experiment.
-
High reproducibility: pooled analysis after labeling minimizes technical variability.
-
Versatile applicability: compatible with diverse biological sample types, including cells, tissues, and biofluids.
-
Ratio compression due to co-isolation and co-fragmentation, which can reduce quantitative accuracy.
-
Relatively high reagent costs.
-
Dependence on high-performance mass spectrometry instrumentation.
-
High proteome coverage achieved through integrated high-pH fractionation and deep MS acquisition strategies.
-
Improved quantitative accuracy via optimized labeling efficiency and robust normalization algorithms.
-
End-to-end bioinformatics support, encompassing differential protein analysis, GO/KEGG enrichment, and protein-protein interaction network construction.
Accurate and high-throughput protein quantification across multiple samples is a fundamental requirement in modern proteomics for elucidating biological variability. Since its introduction in 2004, iTRAQ (Isobaric Tags for Relative and Absolute Quantitation) has become a widely adopted strategy for multiplexed quantitative proteomic analysis. By combining isobaric isotope labeling with mass spectrometry, iTRAQ enables the simultaneous quantification of up to eight samples (iTRAQ 8-plex), offering substantial advantages in biomarker discovery, disease mechanism elucidation, and studies of drug action.
Principles of iTRAQ Technology: Isobaric Labeling Strategy
iTRAQ is a mass spectrometry-based relative quantification approach that relies on isobaric isotope tags. Its defining feature is that, although individual tags generate reporter ions with distinct masses, all labeled peptides share an identical overall mass, thereby maintaining isobaricity.
1. Structural Components of iTRAQ Tags
Each iTRAQ reagent comprises three functional components:
2. Core Principle: Uniform Precursor Mass, Differential Fragment Ions
Due to their isobaric nature, labeled peptides appear as a single precursor ion in MS1 spectra, eliminating signal splitting caused by mass differences. Upon MS2 fragmentation, however, the tags release reporter ions of distinct masses, which encode the relative abundance of the peptide across samples.
iTRAQ Experimental Workflow: From Sample Preparation to Quantification
The standard iTRAQ workflow consists of the following steps:
1. Protein Extraction and Digestion
Proteins are independently extracted from each biological sample and enzymatically digested, typically using trypsin, to generate peptide mixtures.
2. iTRAQ Labeling and Sample Pooling
Each peptide sample is labeled with a uniquely coded iTRAQ reagent. After labeling, all samples are combined into a single pooled mixture.
3. Peptide Fractionation
To enhance proteome coverage, pooled peptides are frequently fractionated using high-pH reversed-phase chromatography prior to LC-MS/MS analysis.
4. LC-MS/MS Acquisition
Peptides are analyzed using high-resolution mass spectrometers (e.g., Orbitrap or Q-TOF systems), enabling simultaneous peptide identification and quantification.
Signal Interpretation: Decoding iTRAQ Reporter Ions
Quantification in iTRAQ experiments relies on reporter ion signals detected in the low-mass region of MS/MS spectra, which directly reflect relative peptide abundance across samples.
1. Reporter Ion Detection
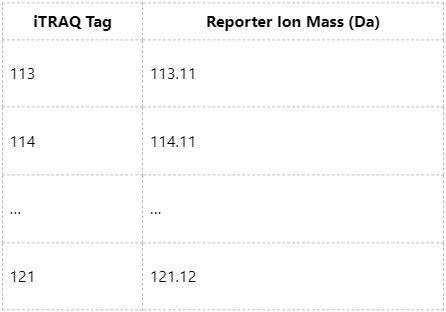
Upon MS2 fragmentation, iTRAQ tags generate reporter ions within the 113-121 Da range. Each tag corresponds to a specific reporter ion mass, as summarized below:
2. Signal Normalization and Quantitative Analysis
Reporter ion intensities, typically represented by peak areas, correspond to peptide abundance in each sample. Quantitative analysis includes:
The resulting quantitative values are subsequently used for statistical analysis and differential expression screening (e.g., fold change > 1.5, p-value < 0.05).
Advantages and Limitations of iTRAQ
1. Advantages
2. Limitations
iTRAQ-Based Proteomics Solutions at MtoZ Biolabs
MtoZ Biolabs offers comprehensive iTRAQ-based quantitative proteomics services supported by advanced Orbitrap platforms and rigorously optimized data analysis pipelines. Key features include:
Despite the growing adoption of next-generation labeling strategies such as TMT, iTRAQ remains a mature and reliable approach in many research contexts. A solid understanding of its underlying principles and signal interpretation is essential for experimental optimization and data quality improvement. With ongoing advances in mass spectrometry technology and computational methods, iTRAQ is expected to continue contributing to high-precision quantitative proteomics. Researchers planning iTRAQ-based studies are encouraged to engage MtoZ Biolabs for professional, integrated experimental and analytical support.
MtoZ Biolabs, an integrated chromatography and mass spectrometry (MS) services provider.
Related Services
How to order?







