Pregelatinized Hydroxypropyl Starch Analysis Service | Pharmaceutical Excipient
- FTIR Spectroscopy: Confirms hydroxypropyl substitution peaks and structural changes due to pregelatinization.
- NMR Spectroscopy: Validates substitution features at the molecular level and evaluates modification degree and consistency.
- Microscopic Observation: Polarized light microscopy or SEM to examine particle morphology and pregelatinization effects.
- Basic Parameters: pH, loss on drying, residue on ignition, particle size distribution, bulk/tapped/true density.
- Residuals and Impurities: Oxidants, sulfites, 1,2-propylene glycol, and other process-related residues.
- Elemental and Microbial Limits: Iron, heavy metals, arsenic, as well as microbial load including total bacteria, yeasts, and molds.
- HPLC/GC: Quantification of key components and impurities.
- Batch Comparisons: Supports formulation screening and consistency evaluation.
- Flowability and Compressibility: Assesses direct compression suitability for tablet development.
- Disintegration and Dissolution: Monitors swelling and disintegration under simulated conditions.
- Controlled Release Evaluation: Examines PHPS as a matrix excipient in modified-release formulations.
- Storage Studies: Monitors changes in physicochemical attributes and flow properties under various temperature/humidity conditions.
- Process Impact: Evaluates effects of manufacturing methods (e.g., spray drying, drum drying) on crystallinity, solubility, and viscosity.
Pregelatinized hydroxypropyl starch (PHPS) is a dual-modified pharmaceutical excipient. During pregelatinization, starch granules are heated and dried to disrupt or partially disrupt their crystalline structure, resulting in excellent cold-water dispersibility and direct compressibility. The hydroxypropyl substitution further enhances solubility, stability, and processing adaptability. With this combined structural modification, PHPS functions simultaneously as a diluent, binder, and disintegrant in tablet and capsule formulations, offering both process flexibility and pharmaceutical functionality.
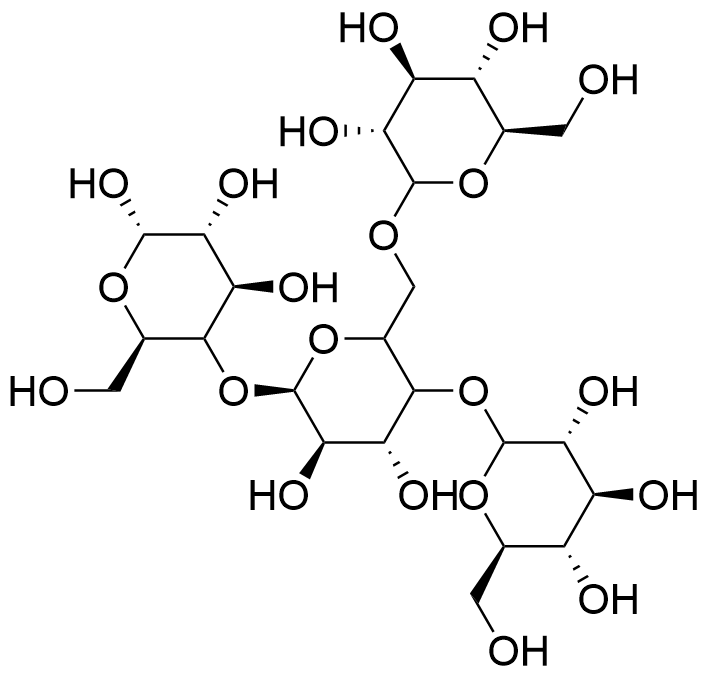
Figure 1. The Structure of Pregelatinized Hydroxypropyl Starch
In modern formulation development, excipients are no longer just fillers or carriers. they directly impact drug stability, bioavailability, and patient compliance. PHPS stands out for its balance of tablet formability and rapid disintegration, performing well in both direct compression and wet granulation processes. As pharmacopeial and regulatory requirements increasingly emphasize excipient safety, batch-to-batch consistency, and functional performance, systematic analysis and quality evaluation of PHPS have become critical.
As a professional pharmaceutical excipient analysis CRO, MtoZ Biolabs leverages advanced analytical platforms and an experienced technical team to provide pregelatinized hydroxypropyl starch analysis service that encompasses evaluations from basic physicochemical properties to functional performance, impurity control, and stability studies, delivering reliable data support for R&D and quality management.
Service at MtoZ Biolabs
MtoZ Biolabs has developed a complete PHPS analytical framework aligned with pharmacopeial standards and tailored to research needs. Our pregelatinized hydroxypropyl starch analysis service covers identification, physicochemical characterization, safety assessment, functional performance, and stability studies, ensuring robust evaluation across the excipient’s lifecycle.
1. Identification and Structural Characterization
2. Physicochemical and Safety Testing
3. Content and Quantitative Analysis
4. Functional Performance and Application Suitability
5. Stability and Hygroscopicity
Analysis Workflow
1. Project Consultation and Scheme Design: Define goals and scope with the client.
2. Sample Reception and Pretreatment: Conduct preliminary inspection and prepare samples.
3. Experimental Implementation: Perform identification, physicochemical, impurity, functionality, and stability testing.
4. Data Review and Interpretation: Compare against pharmacopeial standards and client-specific requirements.
5. Result Delivery: Provide a complete report with conclusions and optional recommendations.
Service Advantages
1. Comprehensive Analytical Framework
We provide a full-spectrum testing framework that covers everything from identification to stability studies, giving you a complete quality profile instead of isolated test results.
2. Alignment with Pharmacopeial Standards
MtoZ Biolabs follow international pharmacopeias and industry guidelines in our pregelatinized hydroxypropyl starch analysis service, ensuring that your results are both scientifically valid and globally acceptable for research and regulatory submissions.
3. Emphasis on Functional Evaluation
We go beyond standard physicochemical data by assessing flow, compressibility, and disintegration in direct compression, wet granulation, and controlled-release contexts, offering practical insights to guide your formulation development.
4. Integration of Stability and Process Impact
MtoZ Biolabs combine stability testing under simulated storage conditions with process background analysis, helping you understand how PHPS performs in real-world applications and where potential risks may arise.
5. Flexible Customization and Efficient Delivery
We tailor every project to your development stage and objectives, offering optimized test packages and timely reporting so you can balance scientific rigor with operational efficiency.
Applications
1. Preformulation Studies: Excipient screening for direct compression and granulation processes.
2. Quality Control: Batch-to-batch consistency evaluation to ensure reliability.
3. Regulatory Submission: Compliance-ready data packages for drug registration.
4. Process Optimization: Verification of PHPS adaptability in new formulations or modified processes.
5. Cross-Industry Use: Exploratory applications in food and cosmetics.
FAQs
Q1: What is the difference between PHPS and pregelatinized starch?
A1: PHPS combines pregelatinization with hydroxypropyl substitution, offering superior cold-water dispersibility, improved stability, and broader applicability in formulations compared with pregelatinized starch.
Q2: What is the typical turnaround time for pregelatinized hydroxypropyl starch analysis service?
A2: Standard analyses usually require 2–3 weeks; inclusion of stability studies may extend the duration.
Sample Submission Suggestions
1. Powdered samples in sealed packaging, protected from moisture and contamination.
2. Recommended quantity ≥50 g; larger amounts needed for functional and stability testing.
3. Room temperature shipping; during humid seasons, include desiccant and secure sealing.
4. Provide background details (source or processing method) if available.
Deliverables
1. Sample reception and inspection records
2. Identification and physicochemical data
3. Impurity and safety test results
4. Functional performance and stability data (if applicable)
5. Comparative analysis against pharmacopeial or in-house standards
6. Comprehensive technical report and conclusions
As a versatile modified excipient, pregelatinized hydroxypropyl starch plays an important role in modern drug formulations. Systematic analysis and quality evaluation are vital not only for ensuring drug safety and efficacy but also for supporting R&D and regulatory compliance.
MtoZ Biolabs, with advanced analytical platforms and an experienced team, provides high-quality pregelatinized hydroxypropyl starch analysis service. Contact us today to obtain tailored solutions and make your R&D and quality management more efficient and reliable.
How to order?







