Pectin Analysis Service | Pharmaceutical Excipient
- Basic parameters such as moisture content, ash value, and pH, used to evaluate the purity and stability of pectin.
- Viscosity and solubility tests to assess rheological behavior in formulations.
- Degree of Esterification (DE): Determines the gelling mechanism and suitable formulation types.
- Galacturonic Acid Content Analysis: A core quality control indicator reflecting purity levels.
- Neutral Sugar Composition Analysis: Using HPLC and GC-MS to determine proportions of arabinose, galactose, rhamnose, etc.
- Heavy metals and arsenic: Measured by AAS or ICP-MS.
- Residual solvents: Analyzed by GC to ensure compliance with pharmacopeial standards.
- Microbial limits: Assessment of bacteria, molds, and yeasts to ensure safety.
- Evaluation of gelling properties and rheology to predict performance in formulations.
- Mucoadhesion and controlled-release performance testing to assess suitability for advanced delivery systems.
- Analytical reports aligned with major pharmacopeias (USP, Ph. Eur., ChP) and industry standards.
- Comparative analysis against market benchmarks to support quality control and formulation optimization.
Pectin is a natural polysaccharide widely present in plant cell walls, mainly composed of galacturonic acid and a small proportion of neutral sugar residues. With unique gelling, thickening, and film-forming properties, pectin has long been used in the food industry. In recent years, with the rapid development of pharmaceutical formulation technologies, the importance of pectin as a pharmaceutical excipient has become increasingly prominent. It can serve as a binder, disintegrant, and controlled-release matrix in tablets, and through crosslinking with other polysaccharides or ions, it can form various drug delivery systems that improve drug stability and targeting. Particularly in controlled-release formulations, orally disintegrating tablets, and mucoadhesive patches, pectin demonstrates great application potential.
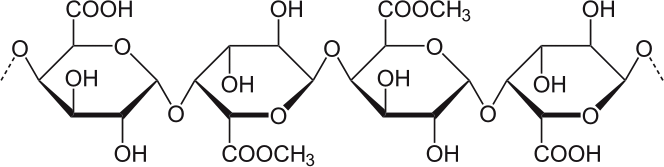
Figure 1. The Structure of Pectin
With the increasing demands of international pharmacopeia standards for excipient quality control, the physicochemical properties, purity, safety, and functional characteristics of pectin require systematic analysis and evaluation. Leveraging advanced analytical platforms and a professional excipient testing team, MtoZ Biolabs offers pectin analysis service for pharmaceutical companies, research institutions, and quality control departments with comprehensive and reliable analytical solutions to ensure the safety and compliance of pharmaceutical formulations.
Services at MtoZ Biolabs
As a natural pharmaceutical excipient, the performance of pectin is significantly influenced by its molecular structure (such as degree of esterification and blockiness), source, extraction process, and purity. MtoZ Biolabs provides the pectin analysis service that range from basic component identification to in-depth structural characterization, covering the following key aspects:
1. Physicochemical Property Testing
2. Structural and Compositional Analysis
3. Impurity and Safety Testing
4. Functional Analysis
5. Comprehensive Evaluation and Standards Comparison
Analysis Workflow
1. Sample Receipt and Registration: Confirm sample status, quantity, and testing requirements.
2. Sample Preparation: Drying, grinding, dissolution, or extraction to ensure suitability for testing.
3. Physicochemical and Structural Analysis: Conducted using multi-platform approaches (chromatography, mass spectrometry, spectroscopy).
4. Safety Testing: Strict evaluation of heavy metals, residual solvents, and microbial limits.
5. Data Integration and Reporting: Comprehensive analytical data, methodology descriptions, and compliance evaluation.
Service Advantages
1. Comprehensive Analytical Coverage
MtoZ Biolabs provides a complete set of tests ranging from basic physicochemical parameters to advanced structural characterization, covering every stage of pectin quality control. Whether verifying excipient source consistency or evaluating functional performance, our platform offers systematic solutions.
2. Standardized Processes
All procedures strictly follow major international pharmacopeias and regulatory guidelines such as ICH Q6B, ensuring authoritative, traceable results that support regulatory submissions and compliance.
3. Multi-Platform Instrumentation
Equipped with advanced LC, GC, MS, and FTIR systems, our laboratory selects the most suitable analytical approach for different pectin types, guaranteeing accuracy and reliability.
4. Experienced Team
Our scientists possess extensive expertise in polysaccharide analysis, pharmaceutical excipient testing, and quality control, enabling us to design tailored solutions for diverse research and formulation needs.
5. One-Time Charge
Our pricing is transparent, with no hidden fees or additional costs.
Applications
1️⃣ Drug Development: Evaluation of excipient performance and optimization in novel formulations.
2️⃣ Quality Control: Ensures consistency and compliance in raw material procurement and production.
3️⃣ Regulatory Submission: Provides pharmacopeia-compliant data for drug registration and approval.
4️⃣ Basic Research: Supports studies exploring the relationship between pectin structure and pharmacological effects.
FAQs
Q: How long does the pectin analysis service take?
Depending on the testing scope, typical turnaround time is 2–4 weeks, with expedited options available upon request.
Q: Can the results be used for drug registration?
Yes. Our reports include methodology descriptions and pharmacopeia-compliant data, suitable for use in pharmaceutical development and regulatory submissions.
Sample Submission Suggestions
1. Sample Type: Powdered pectin or pectin extracts.
2. Sample Amount: At least 10 g recommended to complete all tests.
3. Transport Conditions: Store and ship at ambient temperature, sealed to prevent moisture absorption or contamination.
4. Additional Information: Please provide details such as source, extraction method (if known), and intended application.
Deliverables
1. Comprehensive analytical report
2. Methodology and parameter descriptions
3. Raw data (chromatograms, spectra, etc.)
4. Comparative analysis and conclusions
5. Compliance recommendations and R&D guidance
With a well-established analytical platform and a professional team, MtoZ Biolabs is committed to providing pectin analysis service for clients to ensure the safety and stability of excipients and to deliver robust data support for drug development and registration. Contact MtoZ Biolabs today to accelerate your drug development and excipient quality management with confidence.
How to order?







