Pea Starch Analysis Service | Pharmaceutical Excipient
Pea starch is a natural polysaccharide extracted from peas, mainly composed of amylose and amylopectin. It is characterized by natural origin, high safety, and low allergenicity. Due to its excellent physicochemical properties, such as film-forming ability, gelation, and stability, it is widely used in the field of pharmaceutical excipients. Based on advanced analytical platforms, parameters such as moisture content, starch content, residue content, thermal stability, solubility, and viscosity of pea starch can be analyzed. These parameters can directly reflect its quality and suitability.
Pea starch analysis service based on pharmaceutical excipient has important significance in pharmaceutical applications. By detecting its moisture content and viscosity, its role in tablet forming, capsule filling, and sustained-release formulations can be evaluated. Analysis of residue content and solubility helps ensure excipient purity and formulation stability. In addition, in food and nutritional research, this service can evaluate the functional properties of pea starch to support the development and optimization of functional foods and dietary supplements.
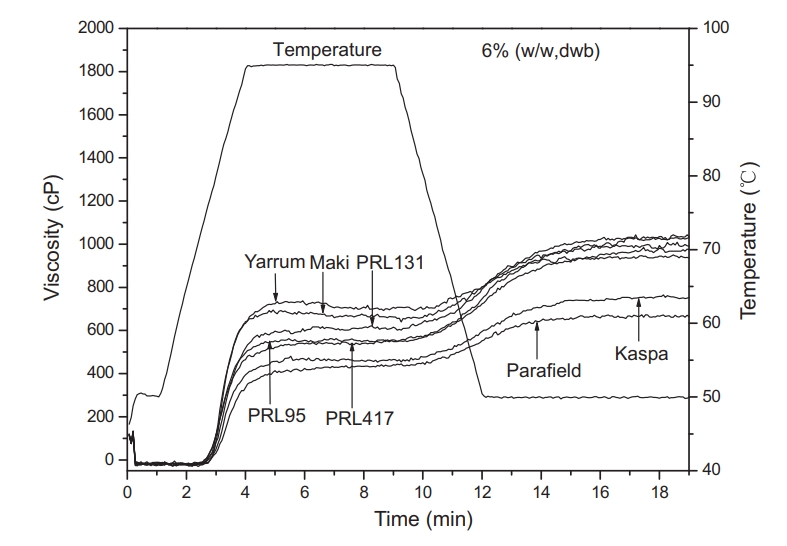
Wang, S J. et al. Food Chemistry, 2011.
Figure 1. RVA Pasting Profiles of Seven Field Pea Starches.
Services at MtoZ Biolabs
Based on advanced analytical platforms, MtoZ Biolabs has launched the pea starch analysis service based on pharmaceutical excipient to comprehensively detect and evaluate characteristics of pea starch. This service relies on multiple analytical techniques, including infrared spectroscopy (IR), high-performance liquid chromatography (HPLC), mass spectrometry (MS), differential scanning calorimetry/thermogravimetric analysis (DSC/TGA), and gravimetric methods, to accurately determine various characteristics of pea starch. Through systematic and multidimensional data support, researchers can gain a full understanding of the purity, stability, and functional properties of pea starch, providing strong assurance for excipient development, formulation optimization, and quality control. MtoZ Biolabs provides services including but not limited to the following:
1. Moisture Content Determination
Moisture content of pea starch is detected using gravimetric methods and infrared spectroscopy (IR) to ensure its stability and storage performance.
2. Starch Content Analysis
High-performance liquid chromatography (HPLC) is applied to quantitatively determine starch components, accurately evaluating their content ratios in the sample.
3. Residue Detection
Mass spectrometry (MS) is used to analyze impurities and residue levels in pea starch, ensuring sample purity and safety.
4. Thermal Stability Evaluation
Differential scanning calorimetry and thermogravimetric analysis (DSC/TGA) are employed to examine the thermal behavior and decomposition characteristics of pea starch under different temperature conditions.
5. Solubility and Viscosity Measurement
Standardized solubility experiments and viscosity tests are performed to systematically evaluate the solubility and rheological properties of pea starch, providing data support for functional studies.
Sample Submission Suggestions
1. Sample Type
Applicable to pharmaceutical excipient samples containing pea starch, which may be in powder, granule, or solution form. The sample source must be clearly identified and representative.
2. Sample Purity
It is recommended to remove impurities or non-target components as much as possible to minimize interference and ensure the accuracy of moisture content, starch content, and other physicochemical parameter analyses.
3. Sample Storage and Transportation
Samples should be stored under dry and light-protected conditions to avoid moisture absorption and degradation. During transportation, sealed packaging is recommended, and low-temperature conditions may be applied if necessary to maintain sample stability.
Service Advantages
1. High-Precision Testing
Leveraging advanced analytical platforms, we precisely assess key indices of pea starch to ensure reliable results.
2. Multidimensional Evaluation
We comprehensively analyze physicochemical properties, thermal stability, and functional characteristics to provide systematic support for excipient development and formulation optimization.
3. Customized Solutions
Based on client needs and sample attributes, we design tailored testing workflows to flexibly meet diverse R&D and quality control objectives.
4. One-Stop Service
From sample handling and index testing to data interpretation and report generation, we deliver end-to-end services that streamline operations and improve research efficiency.
Applications
1. Pharmaceutical Formulation Development
By testing the solubility, viscosity, and moisture content of pea starch, its application value in tablets, capsules, and other solid dosage forms can be assessed, supporting optimization of drug formulation design.
2. Quality Control
Pea starch analysis service can be applied to evaluate the purity, residue levels, and thermal stability of pea starch, ensuring consistency and compliance in pharmaceutical excipient production and application.
3. Excipient Performance Evaluation
Through systematic studies of the functional properties of pea starch, its suitability as a binder, disintegrant, or filler can be validated, supporting drug development and manufacturing.
4. Food and Nutrition Product Research
Pea starch analysis service can be used to assess the physicochemical properties of pea starch in functional foods or nutritional supplements, ensuring formulation stability and product quality.
FAQ
Q1: Why Is It Necessary to Analyze Pea Starch?
A1: As a commonly used pharmaceutical excipient, the moisture content, purity, solubility, and viscosity of pea starch directly affect the stability and release properties of drug formulations. Systematic analysis ensures stable excipient performance and meets formulation requirements.
Q2: What Is the Significance of Thermal Stability Analysis of Pea Starch?
A2: Thermal stability is directly related to the performance of drug formulations during storage and processing. Thermal analysis (DSC/TGA) can evaluate whether the excipient is suitable for long-term preservation and specific processing conditions.
How to order?







