N- and C-Terminal Protein Sequencing Services
- Recombinant Protein Structure Validation
- Biopharmaceutical Quality Control
- Signal Peptide and Processing Site Identification
- Post-Translational Modification Analysis
- Sequence Confirmation of Novel or Unknown Proteins
N- and C-Terminal Protein Sequencing Services refer to specialized analytical services that identify, characterize, and verify the N-terminal and C-terminal sequences of proteins using techniques such as mass spectrometry, and Edman degradation. These services are used not only to confirm the start and stop sites of protein translation but also to identify specific terminal peptides resulting from signal peptide cleavage, terminal modifications, or proteolytic processing.
The N and C-terminal sequences of a protein are critical for determining its biological function, subcellular localization, and stability, and they often play key roles in structural regulation and interaction interfaces. Compared to internal sequences, terminal regions are more susceptible to post-translational modifications (PTMs) such as acetylation, carbamylation, and proteolytic cleavage, which can directly influence protein activity, maturation, and degradation pathways. Precise characterization of terminal sequences is therefore essential in biopharmaceutical development (e.g., recombinant proteins, antibodies, vaccines), protein engineering, quality control, and mechanistic studies.
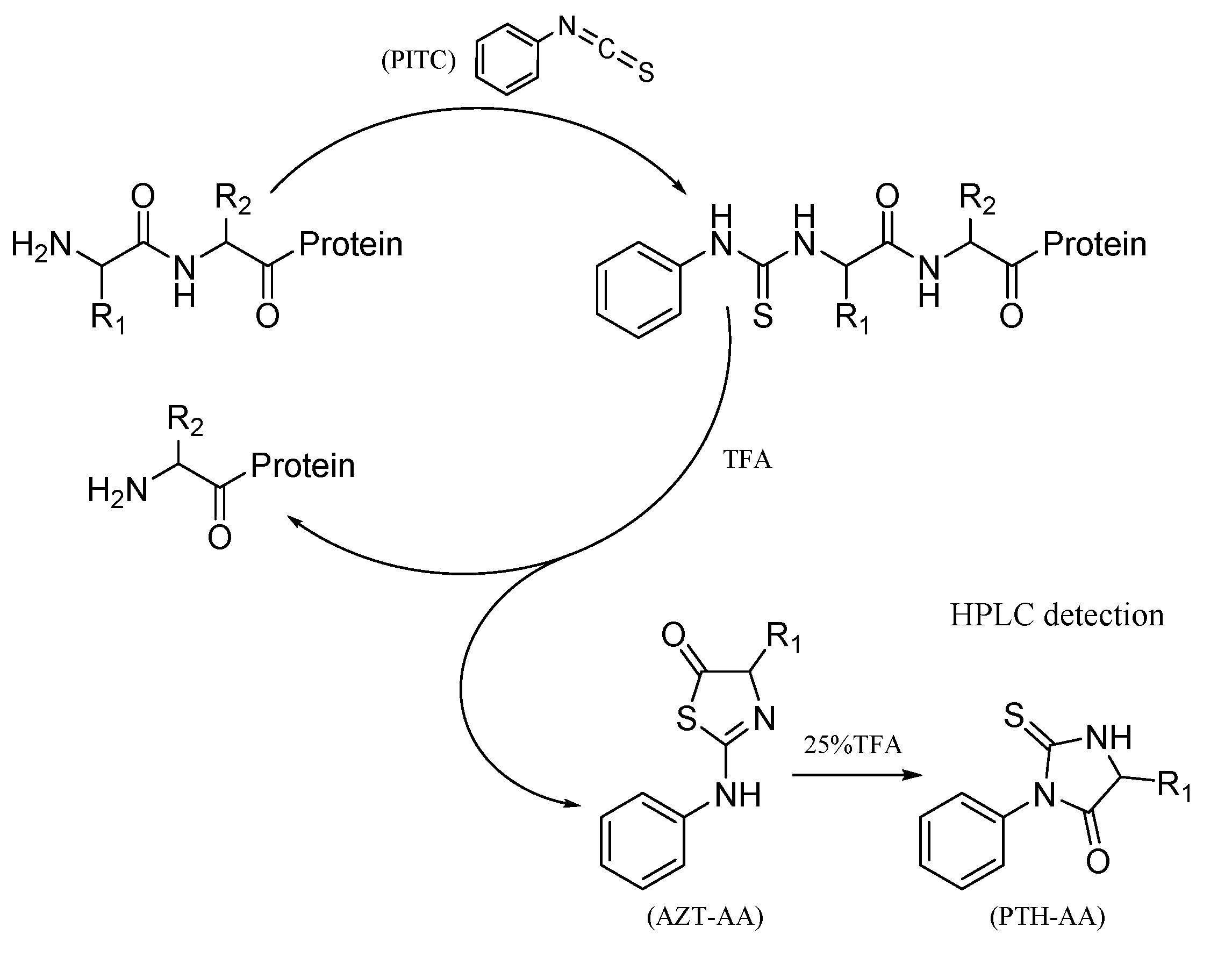
Yang W. et al. Molecules. 2021.
Figure 1. N-terminal sequencing cycle in the Edman degradation assay.
Services at MtoZ Biolabs
Leveraging advanced mass spectrometry platforms, MtoZ Biolabs provides N and C-Terminal Protein Sequencing Services that not only accurately identify protein start and end sequences, but also analyze signal peptide excision sites, fusion tag attachment locations, etc. These services are suitable for a variety of scenarios, including recombinant protein validation, biopharmaceutical consistency evaluation, protein processing mechanism research, and vaccine antigen development.
Identifies the N-terminal sequence of proteins along with associated post-translational modifications. Supports analysis of signal peptide cleavage, verification of fusion tag incorporation, and characterization of N-terminal processing sites.
Characterizes the C-terminal sequence and cleavage sites of proteins. Useful for validating expression integrity and detecting C-terminal modifications.
Protein N-Terminal and C-Terminal Analysis Service
Provides a double-end sequencing solution to obtain protein end sequence, processing status and modification information, fully supporting structure and quality consistency research.
Service Advantages
Advanced Analysis Platform: MtoZ Biolabs established an advanced N and C-Terminal Protein Sequencing Services platform, guaranteeing reliable, fast, and highly accurate analysis service.
One-Time-Charge: Our pricing is transparent, no hidden fees or additional costs.
High-Data-Quality: Deep data coverage with strict data quality control. AI-powered bioinformatics platform integrates all N and C-Terminal Protein Sequencing data, providing clients with a comprehensive data report.
Accurate Cleavage Site Mapping: Enables precise identification of key modifications and processing events such as N-terminal acetylation, signal peptide cleavage and C-terminal truncation, supporting studies on protein function and regulatory mechanisms.
Compatible with Diverse Sample Types: Suitable for a wide range of sample types, including recombinant proteins, native proteins, antibodies, and biopharmaceuticals, meeting the diverse needs of both research and therapeutic development.
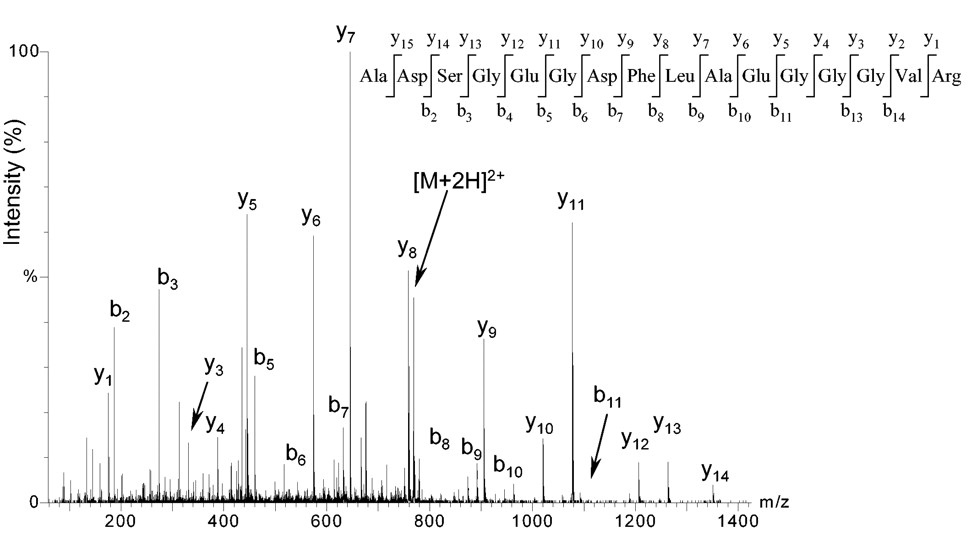
Cristea IM. et al. Blood. 2004.
Figure 2. Sequencing of peptide using MS/MS
Applications
Applications of N- and C-Terminal Protein Sequencing Services include, but are not limited to:
Confirms the accuracy of translation initiation and termination sites, assesses consistency in post-translational processing, and verifies the correct incorporation of fusion tags.
Analyzes terminal structures of bioproducts such as antibodies, vaccines, and enzyme preparations to ensure batch-to-batch consistency and product integrity.
Precisely locates signal peptide cleavage, truncation, or processing sites to support studies on protein maturation and post-translational regulation.
Detects functional modifications such as N-terminal acetylation and C-terminal truncation, helping to elucidate regulatory mechanisms and degradation pathways.
Combines with de novo sequencing to provide essential data for characterizing the terminal sequences and predicting the function of novel or structurally uncharacterized proteins.
Deliverables
1. Comprehensive Experimental Details
2. Materials, Instruments, and Methods
3. Total Ion Chromatogram & Quality Control Assessment (project-dependent)
4. Data Analysis, Preprocessing, and Estimation (project-dependent)
5. Bioinformatics Analysis
6. Raw Data Files
Related Services
How to order?







