Methylcellulose Analysis Service | Pharmaceutical Excipient
MtoZ Biolabs provides Methylcellulose Analysis Service that offers comprehensive testing and systematic evaluation of this widely used non-ionic cellulose ether. Methylcellulose (MC) is produced by etherification of natural cellulose with methoxy groups, typically appearing as a white or off-white fibrous or granular powder. It dissolves readily in water to form a transparent or slightly turbid colloidal solution, while remaining insoluble in ethanol, chloroform, and ether. With its thickening, stabilizing, film-forming, and binding properties, MC plays an important role in pharmaceutical formulations and is also used in food, cosmetics, and building materials. As excipients become increasingly central to drug development and quality control, systematic analysis of MC is essential to ensure consistent quality and regulatory compliance.
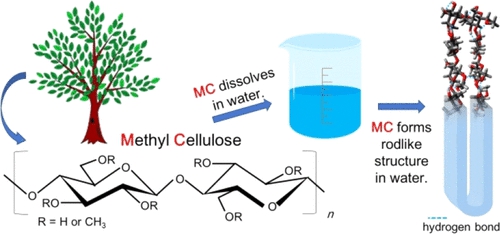
Saiki, E. et al. ACS Omega. 2022.
Figure 1. Methylcellulose Dissolution and Structure Diagram
Services at MtoZ Biolabs
MtoZ Biolabs offers a multi-dimensional evaluation program for MC that spans physicochemical and functional properties:
● Structure and Composition Analysis: Determination of substitution degree and molecular weight distribution using spectroscopic and chromatographic techniques.
● Solubility Testing: Evaluation of solubility performance under various conditions, including clarity and gel characteristics in aqueous systems.
● Viscosity and Rheological Performance: Repeated viscosity testing with viscometers to provide reliable mean values that reflect stability.
● PH and Moisture Assessment: pH measurement and loss on drying under controlled conditions to characterize physicochemical traits.
● Impurity Profiling: Detection of residual solvents, heavy metals, and microbial limits to ensure compliance.
● Functional Property Evaluation: Investigation of thermal gelation, thickening ability, and film-forming capacity to support formulation research.
Why Choose MtoZ Biolabs?
The evaluation of methylcellulose can be technically challenging. The Methylcellulose Analysis Service at MtoZ Biolabs is designed to overcome these barriers by combining advanced instrumentation with established expertise, ensuring reliable, reproducible, and application-ready results.
|
Industry Challenges |
Our Solutions |
|
Testing scattered across platforms, inconsistent results |
Centralized testing with viscometry, IR spectroscopy, chromatography, and microbiological methods, ensuring consistency. |
|
Complex excipient properties not covered by routine methods |
Expertise in excipient analysis, including advanced tests for substitution degree, gelation, and film formation. |
|
Fluctuating results and low comparability |
Standardized workflows with repeated measurements and averaging to improve reliability. |
|
Diverse R&D goals requiring flexible testing |
Customizable testing packages tailored for formulation, process optimization, or batch comparison. |
|
Overly complex reporting difficult to apply |
Clear reports including conditions, data, charts, and concise interpretations designed for practical use. |
Sample Submission Suggestions
To ensure smooth analysis and reliable results, please observe the following:
Sample Types: Powdered methylcellulose, pharmaceutical intermediates, and finished dosage forms are accepted.
Packaging and Storage: Samples should be sealed, protected from moisture and light, and kept away from acids, bases, or corrosives.
Transport Conditions: Ambient conditions are suitable for most samples; desiccants are recommended for long-distance shipping.
Submission Amount: At least 10 g is recommended for standard testing. Please consult us for special project requirements.
MtoZ Biolabs provides detailed submission guidelines to help clients prepare and ship samples according to best practices, ensuring accurate and reproducible outcomes.
By choosing MtoZ Biolabs’ Methylcellulose Analysis Service, clients gain access to complete data covering physicochemical, functional, and stability assessments. This ensures excipients meet compliance requirements while accelerating R&D and market readiness. Beyond methylcellulose, we also analyze a broad range of excipients, including starches, cellulose derivatives, polysaccharides, and other common materials, providing one-stop solutions for pharmaceutical development and quality management.
How to order?







