Method Validation Service | Cryo-EM
In modern pharmaceutical development, method validation is essential to ensure the accuracy, consistency, and regulatory acceptability of analytical techniques. As nanomedicine, gene therapy vectors, and complex biologics continue to evolve, structural characterization plays a critical role in defining product quality. Analytical method validation is a regulatory requirement for drug products, especially those involving structural characterization of nanoscale entities. Reliable methods are required to measure critical quality attributes such as particle morphology, size distribution, aggregation, and encapsulation. Without validated methods, data may lack reproducibility, jeopardizing product approval and market entry. MtoZ Biolabs offers an end-to-end Method Validation Service using Cryo-EM tailored to the unique needs of clients in biopharma and advanced therapy development.
Cryogenic electron microscopy (Cryo-EM), with its ability to directly image nanoscale structures in a near-native state, is increasingly adopted as a powerful tool not only for exploratory research but also for process and quality control. Cryo-EM can provide detailed structural characterization of nanoparticle formulations, viral vectors, liposomes, and lipid nanoparticles (LNPs). By combining high-resolution imaging with statistical analysis, Cryo-EM serves as a quantitative and qualitative platform to validate analytical workflows. MtoZ Biolabs' Method Validation Service leverages Cryo-EM to establish and verify analytical methods tailored for nanoparticle-based drug products, supporting clients from method development through regulatory documentation.
Service at MtoZ Biolabs
MtoZ Biolabs integrates cutting-edge Cryo-EM systems with extensive nanoparticle formulation expertise to deliver actionable structural insights for method validation. Our platform supports developers of gene therapies, mRNA vaccines, protein complexes, and drug-loaded nanoparticles by ensuring that analytical methods are not only scientifically robust but also meet regulatory standards. Our services include:
✅Inter-batch Reproducibility Assessment
Structural validation across multiple production batches to confirm consistency in particle size, morphology, and encapsulation efficiency.
✅Comparability Studies
Head-to-head structural comparison of different formulations, process changes, or manufacturing sites to support formulation bridging or process changes.
✅Stability Assessment
Visualization of morphological integrity and aggregation behavior over time under varied storage or stress conditions, aiding shelf-life justification.
✅In-process Method Verification
Structural monitoring during upstream or downstream processing to ensure control over nanoparticle integrity.
Service Advantages
1. Experienced Team
Our Cryo-EM experts bring extensive experience in nanoparticle characterization and regulatory-focused validation, ensuring scientifically robust and compliant outcomes.
2. End-to-End Service
From method development and execution to final reporting, we offer comprehensive solutions that support clients throughout the formulation and manufacturing lifecycle.
3. Customizable Validation Protocols
We tailor validation plans to meet the specific requirements of your product type, development stage, and regulatory objectives.
4. High-Resolution Cryo-EM Platform
Utilizing state-of-the-art Cryo-EM instruments, we provide near-native imaging to validate key structural attributes such as particle morphology, aggregation, and encapsulation.
5. Standardized Workflows
Our workflows follow rigorously standardized protocols for sample preparation, image acquisition, and data processing to ensure reproducibility and analytical consistency.
Applications
💠Lipid Nanoparticles: Validate methods for measuring LNP morphology, size uniformity, and encapsulation.
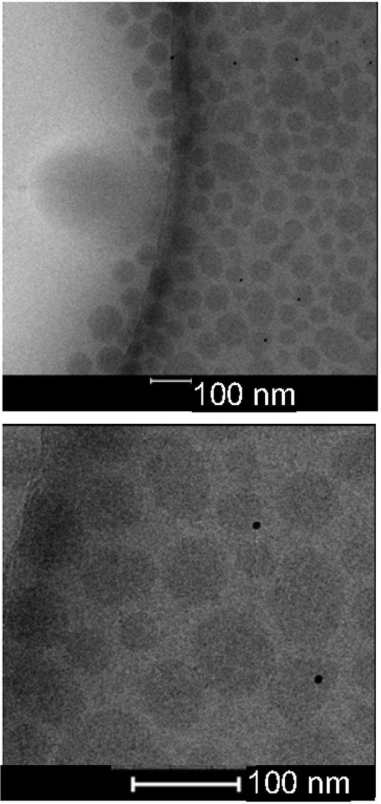
Figure 1. Cryo EM Analysis of siRNA LNP Morphology
💠Viral Vectors (AAV, Lentivirus): Confirm structural integrity, capsid uniformity, and aggregation levels.
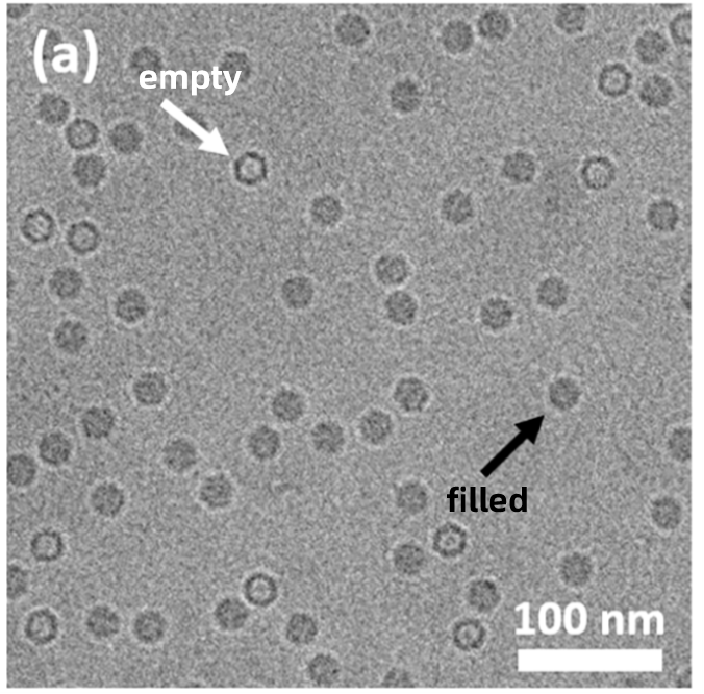
Figure 2. CryoTEM Images of Filled AAV Particles and Empty AAV Particles
💠Protein-Based Nanocarriers: Verify correct assembly, multimerization states, and particle dispersion.
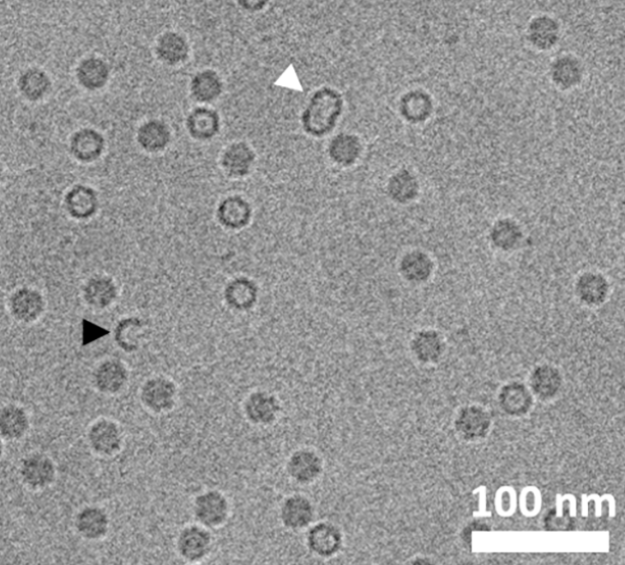
Figure 3. CryoTEM Images of Incomplete Particles (Black Arrowhead) and Improperly Assembled Particles (White Arrowhead)
💠Drug-Loaded Liposomes and Micelles: Support QA for formulation changes and comparability studies.
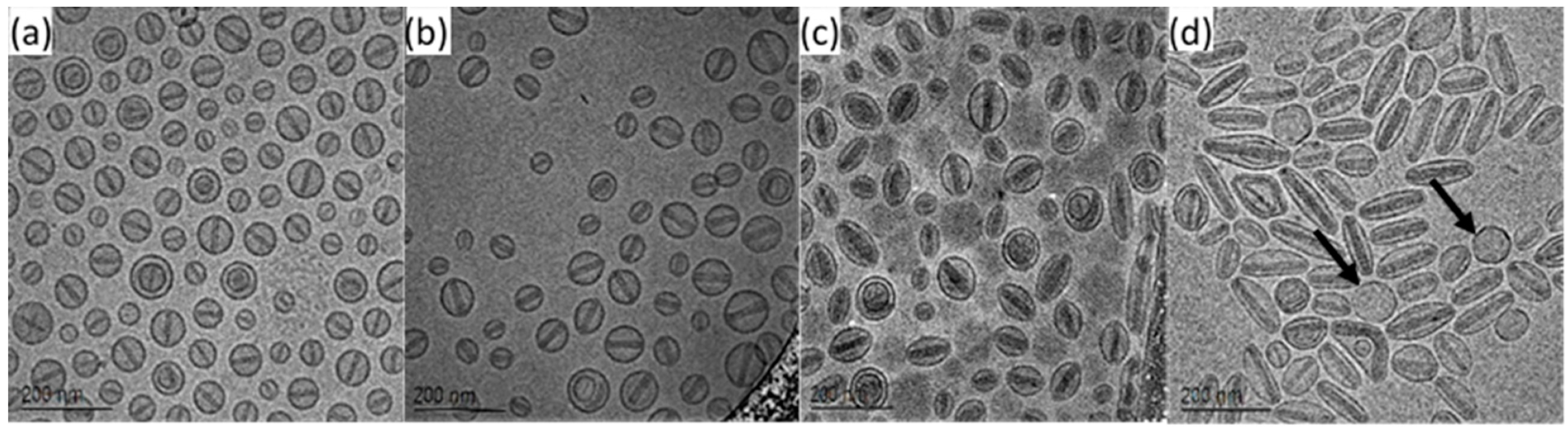
Figure 4. Liposome Elongatedness as a Function of Drug Loading: (a) 1 mg/mL, (b) 2 mg/mL, (c) 3 mg/mL and (d) 4 mg/mL
(Black Arrows Point at Spherical Empty Liposomes)
💠Exosomes and Extracellular Vesicles: Assess sample purity, vesicle size, and morphological consistency.

Figure 5. Cryo-Electron Microscopy Images of Exosomes: Single Vesicles (A); Double Vesicles (B); Double-Membrane Vesicles (C)
FAQ
Q1: How is method reproducibility assessed?
A1: Reproducibility is evaluated by analyzing structural parameters across multiple independent preparations or batches using standardized imaging and analysis protocols.
Q2: Is method validation required for early-phase studies?
A2: While full validation may not be mandatory for early-stage research, many clients choose partial validation to support preclinical decisions and build regulatory readiness early.
As pharmaceutical products grow increasingly complex, the ability to validate analytical methods for structural characterization becomes indispensable. Cryo-EM stands out for its precision, minimal sample perturbation, and unmatched visualization capabilities. MtoZ Biolabs empowers clients with a validated Cryo-EM framework to strengthen product development, streamline regulatory interactions, and accelerate commercialization. To learn more about our Cryo-EM Method Validation Service, or to request a consultation, contact us today.
How to order?







