Melt Flow Index Tester (MFI Tester) Analytical Service
- Wide Applicability: Suitable for various thermoplastic polymers, biodegradable materials, and medical polymers such as syringes and implantable devices.
- Strong Process Relevance: MFI data directly reflect the flow behavior of materials in practical processing such as injection molding, extrusion, and film blowing, providing guidance for process optimization.
- High Data Quality: Reliability of Melt Flow Index Tester (MFI Tester) Analytical Service data is ensured through repeatability testing and strict quality control procedures.
- Customized Service: Testing conditions can be adjusted according to client requirements to support personalized research and quality validation.
Melt Flow Index Tester (MFI Tester) Analytical Service is a professional testing service that measures the mass or volume flow rate of a polymer melt through a standard capillary under specified temperature and load conditions. This service quantitatively characterizes the melt flow index (MFI) of polymers, thereby reflecting their molecular weight level, molecular weight distribution, and processing adaptability in the molten state.
MFI, also known as the melt flow rate (MFR), is a key parameter for evaluating the flowability of thermoplastic plastics and medical polymers during melt processing. Since the establishment of relevant standards by ASTM, this parameter has become a critical reference for polymer material research and development, quality control, and process optimization. The value of MFI directly influences the processability of materials in injection molding, extrusion, blow molding, film preparation, and drug sustained-release carrier formation, as well as the performance of the final product.
Technical Principles
MFI testing is based on the principle of capillary extrusion flow. Polymer granules are heated and melted at a set temperature and extruded through a capillary with a diameter typically of 2.1 mm under a specified load. By measuring the mass of the sample extruded within 10 minutes and expressing the result in g/10 min, the MFI is obtained. A higher MFI value indicates that the polymer has a relatively lower molecular weight, lower melt viscosity, better flowability, and superior processing performance. A lower MFI value indicates that the polymer has a relatively higher molecular weight, higher melt viscosity, poorer flowability, but often better mechanical strength and durability.
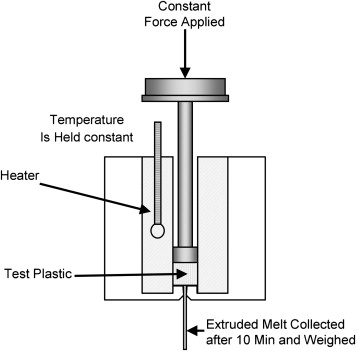
Mckeen L. Effect of Sterilization Methods on Plastics & Elastomers. 2018.
Figure 1. Melt Flow Index Test Apparatus
Services at MtoZ Biolabs
MtoZ Biolabs provides Melt Flow Index Tester (MFI Tester) Analytical Service to accurately measure the melt flow rate (MFR/MFI) of thermoplastic materials under high-temperature conditions, comprehensively reflecting the flowability and processing adaptability of polymers. This service is widely applicable to the quality evaluation and process optimization of polymer raw materials, biodegradable materials, and medical polymers such as syringes and implantable devices, offering scientific support for research, development, and production.
Analysis Workflow
The general process of Melt Flow Index Tester (MFI Tester) Analytical Service is as follows:
1. Sample Preparation
Pre-treat the sample according to its form (granules, powder, or liquid) to ensure homogeneity and remove impurities.
2. Instrument Preheating
Heat the melt flow rate tester to the specified test temperature and maintain a constant temperature to stabilize the system.
3. Sample Loading and Melting
Place the sample into the heating barrel and allow it to melt completely and stabilize under constant temperature conditions.
4. Load Application
Apply the specified load using a standard piston to extrude the sample through the capillary orifice.
5. Flow Measurement
Collect and weigh or measure the amount of sample extruded within a set period of time, typically 10 minutes.
6. Data Calculation
Convert the measurement to obtain the melt flow rate (MFI, expressed in g/10 min). If necessary, repeat the test under different temperatures and loads to evaluate the rheological properties of the material.
7. Result Reporting
Provide a complete report including test data and flowability evaluation, with interpretation based on standards or client requirements.
Service Advantages
Sample Submission Suggestions
1. Sample Types
Granule, powder, or liquid samples are all acceptable.
2. Storage and Transportation
Samples should be sealed to prevent moisture absorption and contamination. Temperature-sensitive samples are recommended to be transported under low-temperature conditions.
It is recommended to contact the MtoZ Biolabs technical team prior to sample submission to obtain detailed and tailored guidelines for sample preparation and submission.
Applications
1. Polymer Research and Modification
Use MFI values to reflect molecular weight and distribution differences, providing structure–property correlation data for the design and modification of novel polymers.
2. Pharmaceutical and Medical Materials
Evaluate the flowability and processing adaptability of pharmaceutical excipients and medical polymers such as syringes and implantable materials.
3. Quality Control
Monitor MFI differences among different batches of samples to ensure production stability and traceability.
How to order?







