Maltodextrin Analysis Service | Pharmaceutical Excipient
- Sample Type: Powder or granular maltodextrin
- Minimum Quantity: 5 grams per sample
- Storage Conditions: Store in sealed containers at room temperature in a dry environment
- Additional Documentation: Provide lot number, supplier details, and intended application
MtoZ Biolabs provides Maltodextrin Analysis Service for the precise characterization and quality evaluation of maltodextrin used in pharmaceutical and nutritional formulations. Maltodextrin is a partially hydrolyzed starch polysaccharide produced by enzymatic or acid treatment of natural starch sources such as corn, rice, or potato. Its performance as an excipient depends on parameters such as dextrose equivalent (DE) value, molecular weight distribution, solubility, and purity.
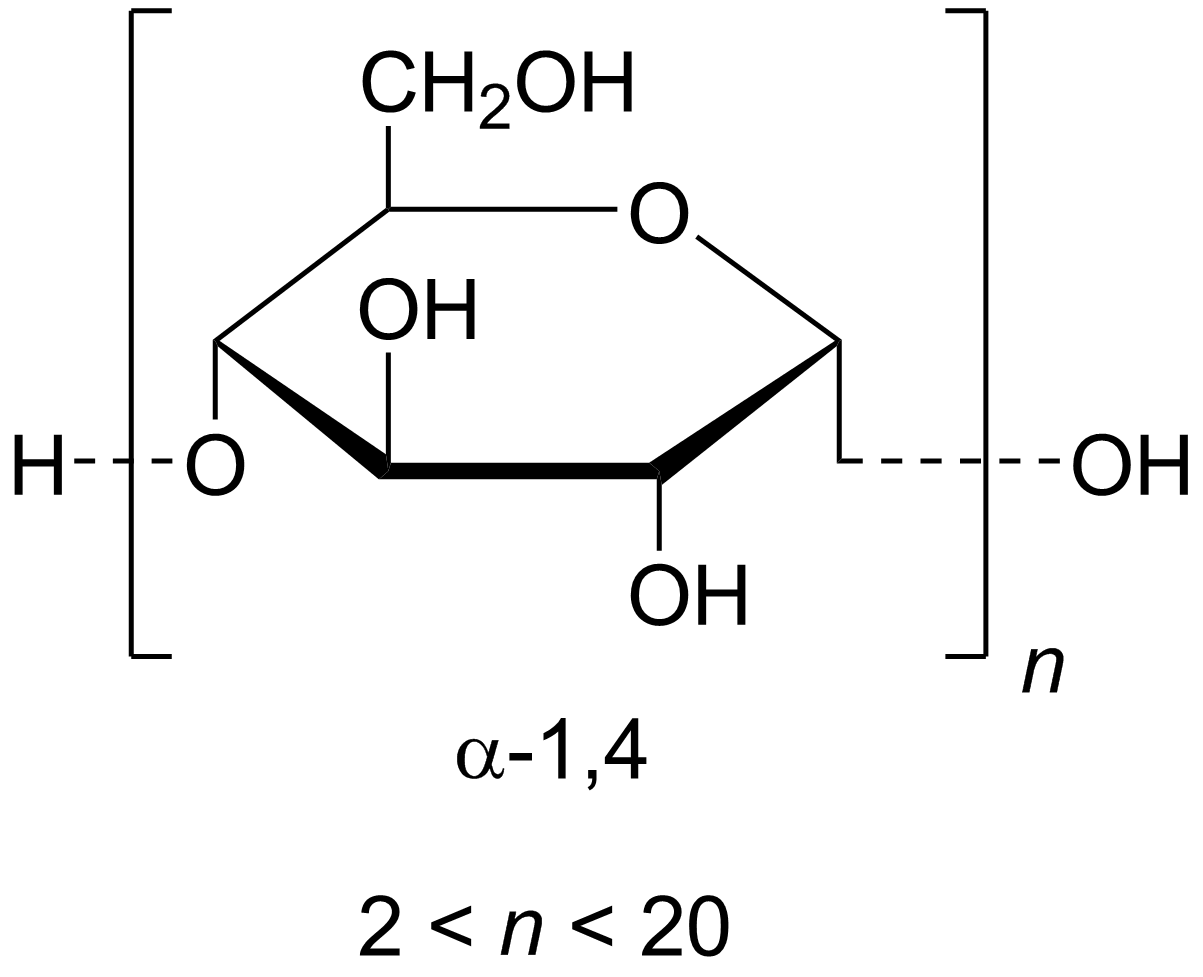
Figure 1. Molecular Structure of Maltodextrin
As a versatile ingredient, maltodextrin is widely used as a binder, filler, stabilizer, and carrier in solid and liquid dosage forms. MtoZ Biolabs utilizes advanced analytical platforms and validated laboratory methods to ensure high-precision testing, supporting clients in maintaining formulation consistency, ensuring compliance, and improving product performance. Our Maltodextrin Analysis Service provides comprehensive data to help optimize formulation strategies and confirm excipient integrity.
Services at MtoZ Biolabs
MtoZ Biolabs offers a full suite of validated analytical methods designed to assess the chemical, physical, and microbiological properties of maltodextrin. Each method follows strict quality standards to ensure reliable, reproducible, and regulatory-compliant results.
● Identification
Sample identity is confirmed using FTIR spectroscopy and chemical reactivity testing. When the sample reacts with alkaline copper tartrate to produce a red precipitate, it confirms the reducing saccharide structure of maltodextrin.
● Dextrose Equivalent (DE) Determination
The DE value, which indicates the degree of starch hydrolysis, is measured by titration and spectrophotometric analysis. This value helps evaluate sweetness, viscosity, and chain length distribution.
● Moisture and Loss on Drying
Moisture content is measured using gravimetric and Karl Fischer titration methods to ensure product stability and prevent microbial contamination during storage.
● pH and Acidity Measurement
Aqueous maltodextrin solutions are analyzed using a calibrated pH meter. A typical acceptable range between 4.5 and 6.5 ensures optimal chemical compatibility and formulation performance.
● Protein Content Determination
Protein levels are quantified through nitrogen analysis, ensuring that residual protein levels remain within acceptable limits and verifying the purity of the raw material.
● Sulfur Dioxide Analysis
Sulfur dioxide concentration is determined using iodometric titration. This test ensures that residual processing reagents are below safety thresholds and meet excipient-grade purity requirements.
● Heavy Metal and Arsenic Testing
ICP-MS and colorimetric assays are used to detect trace heavy metals and arsenic. These tests confirm compliance with pharmaceutical safety standards for excipient materials.
● Solubility and Insoluble Residue Testing
Water solubility and filtration analysis determine the presence of insoluble particles. High-quality maltodextrin should have an insoluble residue content of less than 1.0 percent.
● Microbial Limit Testing
Microbial enumeration and total viable count analyses are performed to verify product safety and compliance with microbiological quality standards.
Through these analytical techniques, MtoZ Biolabs’ Maltodextrin Analysis Service delivers detailed quality insights that support excipient evaluation, batch consistency testing, and formulation development.
Sample Submission Suggestions
Detailed submission guidelines are available upon request to ensure accurate testing and reporting.
Why Choose MtoZ Biolabs?
✅ Expert Analytical Team: Specialists in carbohydrate and excipient characterization
✅ Comprehensive Analytical Coverage: Multi-parameter testing for complete quality evaluation
✅ Validated and Reproducible Methods: Standardized procedures ensuring consistent results
✅ Advanced Instrumentation: Equipped with FTIR, HPLC, ICP-MS, and titration systems
✅ Regulatory-Ready Documentation: Reports formatted for QA and compliance submissions
The Maltodextrin Analysis Service from MtoZ Biolabs is applicable across pharmaceutical, food, and nutritional industries, supporting excipient verification, formulation optimization, and product stability evaluation. Reliable analytical data generated by our service ensures material consistency, regulatory compliance, and batch-to-batch reproducibility.
By integrating scientific expertise with state-of-the-art analytical technologies, MtoZ Biolabs delivers accurate and actionable results for every client. To discuss your testing needs or request a customized solution, contact MtoZ Biolabs for professional analytical support tailored to your formulation and quality assurance goals.
FAQ
Q1: How Is the DE (Dextrose Equivalent) Value Measured?
A1: To determine the DE value, maltodextrin solution (with methylene blue as the indicator) is titrated using alkaline copper tartrate solution until the blue color disappears. A glucose solution is analyzed in parallel as a control, and the DE value is calculated based on the titrant volume consumed. This parameter indicates the degree of hydrolysis and correlates with the sweetness, solubility, and molecular structure of maltodextrin.
How to order?







