Lipid Nanoparticles (LNPs) Structure Characterization Service | Cryo-EM
Lipid nanoparticles (LNPs) structure characterization is a high-resolution structural analysis based on cryogenic electron microscopy (Cryo-EM), specifically designed to analyze key features of lipid nanoparticles (LNPs), including their three-dimensional structure, particle size distribution, internal vesicle architecture, and lipid layer arrangement. This technique involves rapidly freezing LNPs samples to liquid nitrogen temperatures to prevent denaturation or collapse under ambient or vacuum conditions. High-resolution imaging is then performed under low-dose conditions, preserving the native structure, making it suitable for lipid systems sensitive to structural disruption.
Lipid nanoparticles (LNPs) structure characterization service is widely applied in cutting-edge biomedical fields such as mRNA vaccines, nucleic acid drug delivery systems, targeted delivery carriers, and liposomal drug development. It helps researchers evaluate the structural stability, delivery efficiency, and mechanism of drug delivery systems. With Cryo-EM’s unique advantages in label-free, low-interference imaging, this service has become a critical structural analysis tool in lipid nanoparticle-based drug development.
Services at MtoZ Biolabs
Based on a high-end cryogenic transmission electron microscopy platform, the lipid nanoparticles (LNPs) structure characterization service based on Cryo-EM provided by MtoZ Biolabs enables rapid freezing and native-state fixation of lipid nanoparticle samples at ultra-low temperatures, allowing for high-resolution imaging without staining and under low-dose conditions. This service delivers critical information such as the three-dimensional structure, particle size distribution, internal vesicle architecture, lipid bilayer arrangement, and payload distribution of LNPs. With automated image acquisition and precise reconstruction, the results achieve sub-nanometer spatial resolution and high structural fidelity, making the service ideal for structural evaluation and mechanistic studies of mRNA vaccines, nucleic acid therapeutics, and targeted nanomedicine systems.
Analysis Workflow
1. Sample Vitrification
LNPs samples are rapidly cooled to liquid nitrogen temperature to form vitreous ice, preserving their native structure.
2. Cryo-EM Image Acquisition
High-resolution, low-dose, multi-angle images are collected using a cryogenic transmission electron microscope to minimize sample damage.
3. Image Preprocessing and Particle Identification
Images are denoised, corrected, and screened to select qualified particles, laying the foundation for reconstruction.
4. 3D Reconstruction and Structural Analysis
Reconstruction algorithms are applied to generate 3D structural images of LNPs, revealing features such as vesicle architecture and lipid layer distribution.
5. Data Delivery and Report Generation
Original images, structural models, and a professional analytical report are provided to support the development and optimization of drug delivery systems.
Service Advantages
1. Native-State Imaging with Structural Fidelity
LNPs are vitrified under ultra-low temperature conditions to prevent deformation, preserving their natural structure.
2. Nanometer Resolution
Powered by advanced Cryo-EM platforms such as Titan Krios, the service enables visualization of fine features like lipid bilayers and vesicle architecture.
3. Support for Diverse LNP Types
Applicable to structural analysis of various LNP systems, including mRNA carriers, siRNA delivery vehicles, and liposomal drugs.
4. Non-Staining, Low-Dose Operation
No negative staining is required, minimizing interference and ensuring both structural integrity and high image contrast.
Applications
1. Structural Evaluation of mRNA Vaccine Carriers
The lipid nanoparticles (LNPs) structure characterization service can be used to analyze encapsulation efficiency, lipid distribution, and vesicle architecture in mRNA vaccines, ensuring delivery performance and formulation stability.
2. Development of Nucleic Acid Drug Delivery Systems
By examining the structural features of carriers for siRNA, miRNA, and other nucleic acids, the service supports optimization of delivery efficiency and cellular uptake.
3. Verification of Liposomal Drug Formulations
The lipid nanoparticles (LNPs) structure characterization service enables structural integrity and size uniformity assessment of multilamellar or unilamellar liposomes, providing a basis for quality control in liposomal drugs.
4. Structure–Function Analysis of Nanomedicines
Comprehensive analysis of morphology, lamellar structure, and internal spatial distribution allows exploration of the relationship between nanoparticle structure and therapeutic efficacy.
5. Screening of Novel Nanocarrier Materials
The lipid nanoparticles (LNPs) structure characterization service can evaluate the structural stability and encapsulation mechanisms of emerging LNP materials, such as ionic liquid lipids and pH-responsive lipids.
Case Study
1. Analysis of Lipid Nanoparticles by Cryo-EM for Characterizing siRNA Delivery Vehicles
This study aims to quantitatively characterize lipid nanoparticles (LNPs) used for siRNA delivery through a semi-automated cryogenic electron microscopy (Cryo-EM) image analysis framework. The subjects are two types of siRNA-loaded LNPs that differ only in component ratios, both composed of cationic lipids, cholesterol, and polyethylene glycol (PEG)-modified lipids. The authors performed Cryo-EM imaging of the LNPs and applied processing techniques including image enhancement, target segmentation, and attribute quantification to extract key parameters such as particle size, morphology, and lamellar structure. The results demonstrate that Cryo-EM analysis not only provides reliable data on particle size and shape but also detects small-size populations that standard dynamic light scattering (DLS) fails to identify, revealing the heterogeneity within LNP populations. The study concludes that this semi-automated Cryo-EM image analysis approach significantly enhances the quantitative assessment of LNP structural features and serves as a powerful tool for the design and evaluation of optimized siRNA delivery systems.
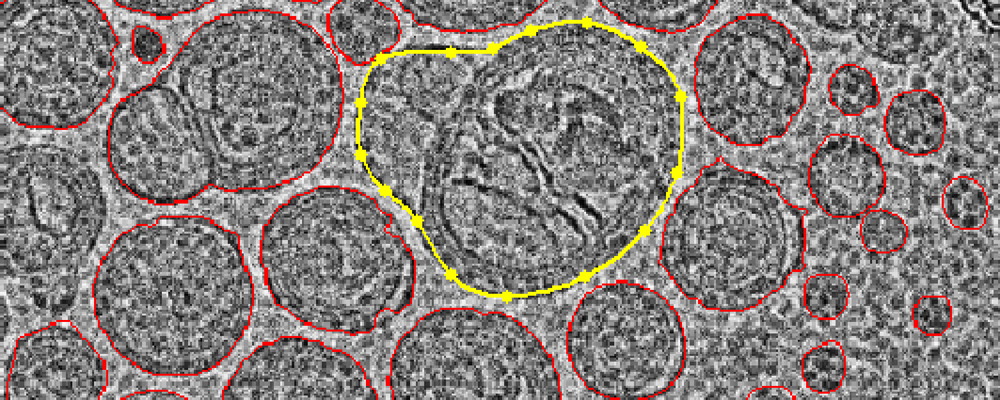
Crawford, R. et al. International Journal of Pharmaceutics, 2011.
Figure 1. The Graphical Abstract of this Study.
2. Impact of Non-Ionizable Lipids and Phase Mixing Methods on Structural Properties of Lipid Nanoparticle Formulations
This study aims to evaluate the impact of non-ionizable lipids and different phase mixing methods on the structural properties of lipid nanoparticles (LNPs), with a particular focus on the role of cryogenic electron microscopy (Cryo-EM) in structural characterization. The research subjects are various siRNA-loaded LNP formulations prepared using two distinct mixing techniques: microfluidics and solvent injection. Cryo-EM was employed to visualize the morphology and internal structure of the LNPs. The results show that LNPs prepared by solvent injection generally exhibit well-defined spherical morphology and distinct multilamellar structures with an interlayer spacing of approximately 5 nm, whereas LNPs produced by microfluidics are more heterogeneous, displaying irregular shapes and fewer discernible lamellar features. Cryo-EM imaging clearly reveals the microstructural differences of LNPs under varying formulation conditions. The study concludes that Cryo-EM is an effective tool for uncovering nanoscale structural variations in LNPs, thereby facilitating formulation optimization and enhancing the stability and performance of nucleic acid delivery systems.
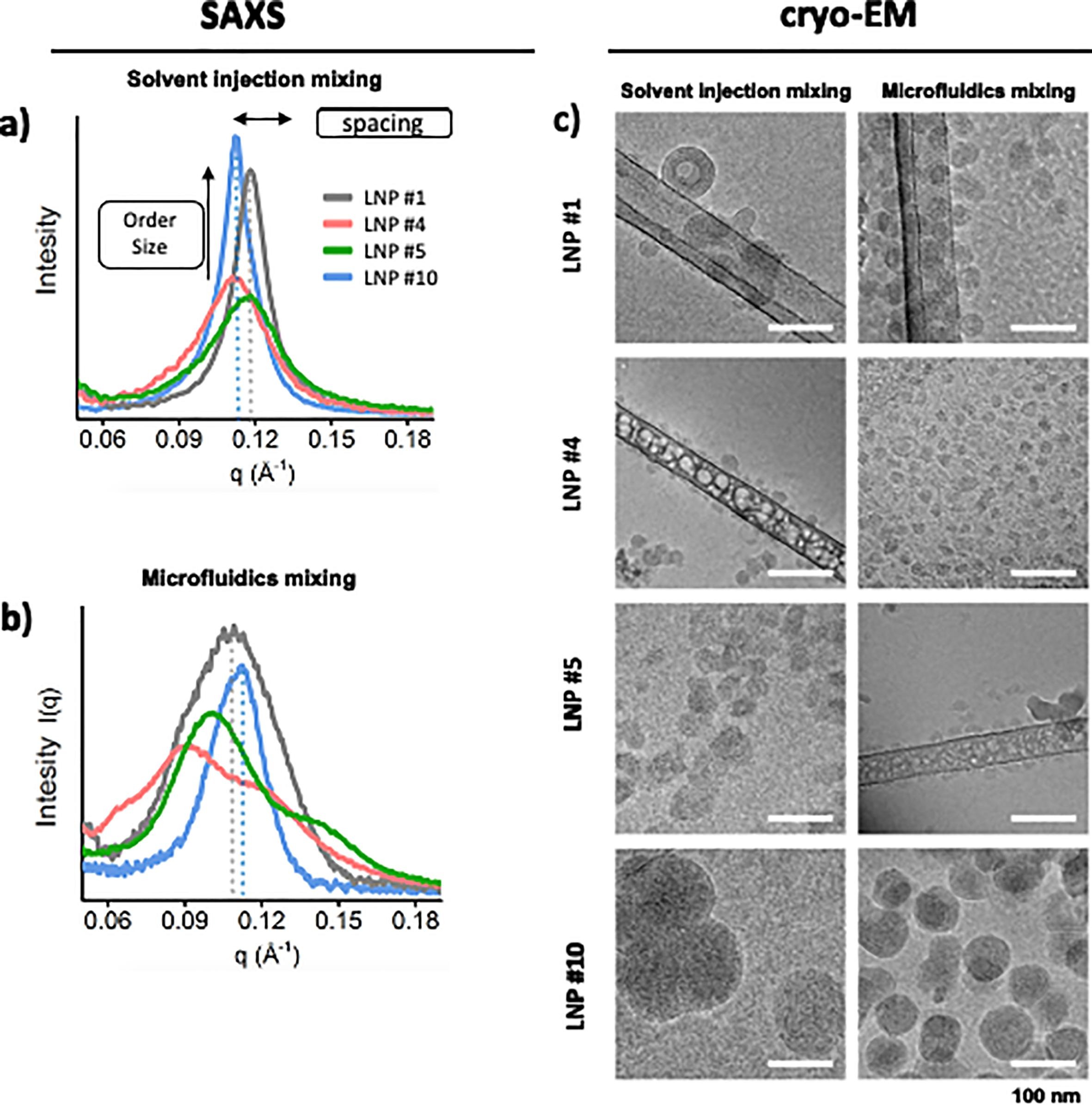
Pratsinis, A. et al. International Journal of Pharmaceutics, 2023.
Figure 2. ASO-Lipid Compartmentalization Shown by SAXS and Cryo-EM.
FAQ
Q1: What Are the Advantages of Using Cryo-EM to Analyze LNPs?
A1: Cryo-EM enables native-state imaging without the use of stains and under low-dose electron beam conditions, preventing structural denaturation. It faithfully captures the vesicle architecture, lipid layer arrangement, and payload distribution of LNPs, making it ideal for nanosystems with fine structures and environmental sensitivity.
Q2: Does the Service Support Analysis of Different Ttypes of LNPs?
A2: Yes. This service is applicable to various LNP systems, including mRNA carriers, siRNA delivery platforms, liposomes, and multi-component lipid nanoparticle formulations.
Q3: Does Cryo-EM Imaging Damage the Structure of LNPs?
A3: No. By employing rapid vitrification and low-dose imaging, Cryo-EM preserves the native structure of LNPs with minimal interference, ensuring accurate structural analysis.
How to order?







