Iodination Proteomics Service
Iodination proteomics analysis is a proteomics approach based on high-resolution mass spectrometry technology, specifically designed to detect and characterize protein iodination modifications. It enables researchers to gain deeper insights into the regulatory roles of iodination in both physiological and pathological processes. Iodination refers to the covalent attachment of iodine atoms to amino acid residues such as tyrosine, altering protein structure, function, and signaling activity. By combining specific enrichment strategies with highly sensitive mass spectrometry platforms, this service allows for comprehensive identification of iodination sites and corresponding peptide sequences.
Iodination proteomics service is widely applied in biomedical fields such as thyroid disease mechanism studies, radiation biology, environmental toxicology, iodine-related metabolic research, and immune regulation. It is also suitable for biomarker discovery, food safety testing, and the evaluation of iodinated drug modifications, supporting both scientific research and industrial translation.
Services at MtoZ Biolabs
Based on advanced analytical platforms and optimized sample preparation workflows combined with specific iodinated peptide enrichment strategies, the iodination proteomics service provided by MtoZ Biolabs enables highly sensitive identification of iodination sites, peptide sequences, and modification intensity in proteins. This service is applicable to complex samples such as cells and tissues and delivers high-quality data including modification sites, peptide sequences, spectral information, and relative quantification. It supports researchers in exploring the functional roles of iodination modifications in both physiological and pathological processes. The analytical methods for iodination include, but are not limited to, the following:
Analysis Workflow
1. Protein Extraction and Digestion
Total proteins are extracted from cell or tissue samples, followed by reduction, alkylation, and enzymatic digestion to preserve iodination modifications, laying the foundation for downstream detection.
2. Enrichment of Iodinated Peptides
Iodinated peptides are selectively enriched using specific chemical labeling or anti-iodination antibodies, enhancing the detection of low-abundance modified peptides.
3. Multi-Modal Detection Approaches
Depending on experimental needs, various methods such as mass spectrometry, radioactive/non-radioactive labeling, and Western blotting can be employed to comprehensively identify iodination sites, peptide sequences, and expression levels.
4. Data Analysis and Result Reporting
Through database searching and computational analysis, the service provides information on modification sites, peptide sequences, quantitative data, and functional annotations to support downstream mechanistic studies and target discovery.
Sample Submission Suggestions
1. Sample Types
A wide range of sample types is supported, including cells, tissues, and purified proteins. It is recommended to use samples subjected to iodination treatment or with active iodination processes to enhance modification detection efficiency.
2. Buffer System
Avoid buffer solutions containing high salt, SDS, strong reducing agents, or iodination inhibitors, as they may interfere with modification stability or mass spectrometry compatibility. Buffer optimization guidance is available upon request.
3. Sample Transport
Samples should be stored at –80°C and shipped on dry ice to ensure the stability of iodination modifications and maintain overall protein integrity.
Service Advantages
1. Multi-Strategy Detection Integration
Combining mass spectrometry, radioactive/non-radioactive labeling, and Western blotting, this service accommodates diverse research needs and supports both global screening and targeted validation.
2. Specific Enrichment Approach
Targeted enrichment is achieved using chemical labeling or anti-iodinated peptide antibodies, significantly improving the detection efficiency and specificity of low-abundance iodination modifications.
3. One-Time-Charge
Our pricing is transparent, no hidden fees or additional costs.
4. Comprehensive Technical Support
From sample preparation and modification detection to data analysis and annotation, the service offers a complete workflow to facilitate mechanistic studies, disease-related investigations, and biomarker discovery.
Applications
1. Thyroid Function and Iodine Metabolism Research
The iodination proteomics service can be used to investigate the role of iodination in thyroid hormone synthesis and regulation, helping to elucidate molecular mechanisms associated with abnormal iodine intake.
2. Radiation Stress and Environmental Exposure Studies
This service is suitable for assessing protein iodination level changes following radioactive iodine exposure, revealing the impact of radiation on cellular function and signaling pathways.
3. Disease Mechanism Exploration
Iodination proteomics can be applied to study the regulatory significance of iodination modifications in autoimmune diseases, cancers, and metabolic disorders.
4. Drug Evaluation and Biomarker Development
The service supports target validation and mechanism analysis of iodinated drugs or radiotherapeutics, facilitating the discovery of novel biomarkers and advancing personalized medicine.
Case Study
1. Measurements of Iodination in Thyroglobulin: A Step Toward the Next Generation of Thyroid Cancer Monitoring
This study aimed to explore the use of iodination proteomics analysis to assess the iodination status of thyroglobulin (Tg) as a means to advance thyroid cancer monitoring strategies. The research focused on commercially sourced human Tg samples, and deep proteomic analysis was conducted using liquid chromatography-tandem mass spectrometry (LC-MS/MS), achieving over 90% sequence coverage of Tg and successfully identifying multiple known and novel iodination sites. Targeted quantitative analysis further confirmed that hormone synthesis predominantly occurs at two key sites, Y24 and Y2766, with their iodination levels serving as potential functional indicators. The study demonstrates that this method offers high resolution and specificity, providing a new tool for routine evaluation of Tg iodination status and distinguishing between functional and non-functional Tg. It holds promise for postoperative monitoring and personalized management of thyroid cancer.
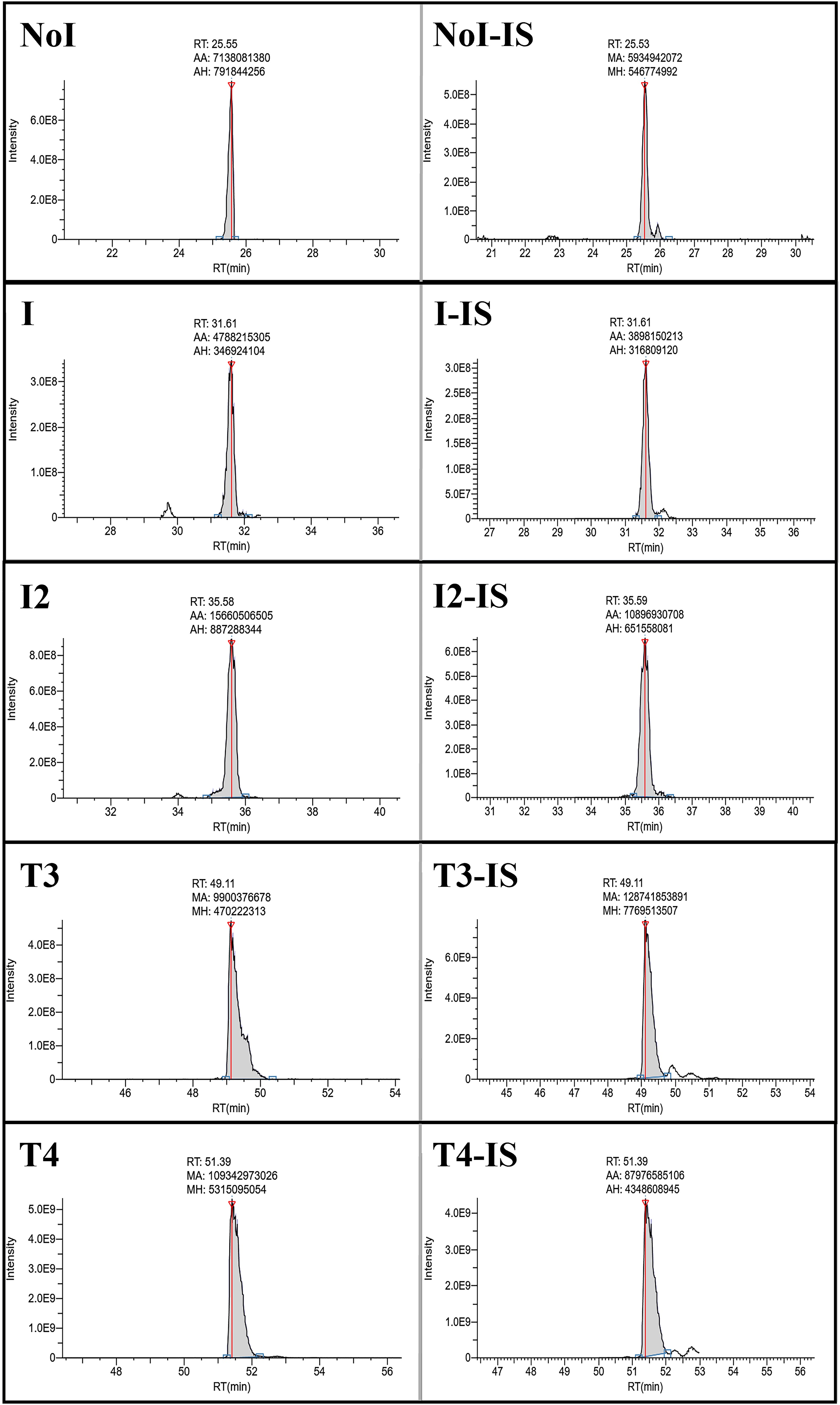
Maus, A. et al. Journal of the Endocrine Society, 2025.
Figure 1. Representative Chromatograms for the Different Iodination States of the Y2573 Position.
2. Revisiting Iodination Sites in Thyroglobulin with an Organ-oriented Shotgun Strategy
This study aimed to systematically analyze iodination sites in mouse thyroglobulin (Tg) using an organ-oriented shotgun proteomics strategy and to explore their role in thyroid hormone synthesis. Multiple enzymatic digestion approaches were combined with a high-resolution LTQ-Orbitrap XL mass spectrometry platform to perform deep analysis of unpurified mouse Tg samples. A total of 37 iodinated tyrosine residues were identified—significantly more than previously reported in human Tg—including four hormonogenic sites and two donor sites, the latter confirmed by detecting tyrosine residues substituted by pyruvate. The study also identified Tg cleavage sites mediated by endogenous proteases. These findings demonstrate that this strategy enables high-coverage iodination proteomics analysis without the need for protein purification, providing a powerful tool for future Tg iodination monitoring and thyroid disease research in human clinical samples.
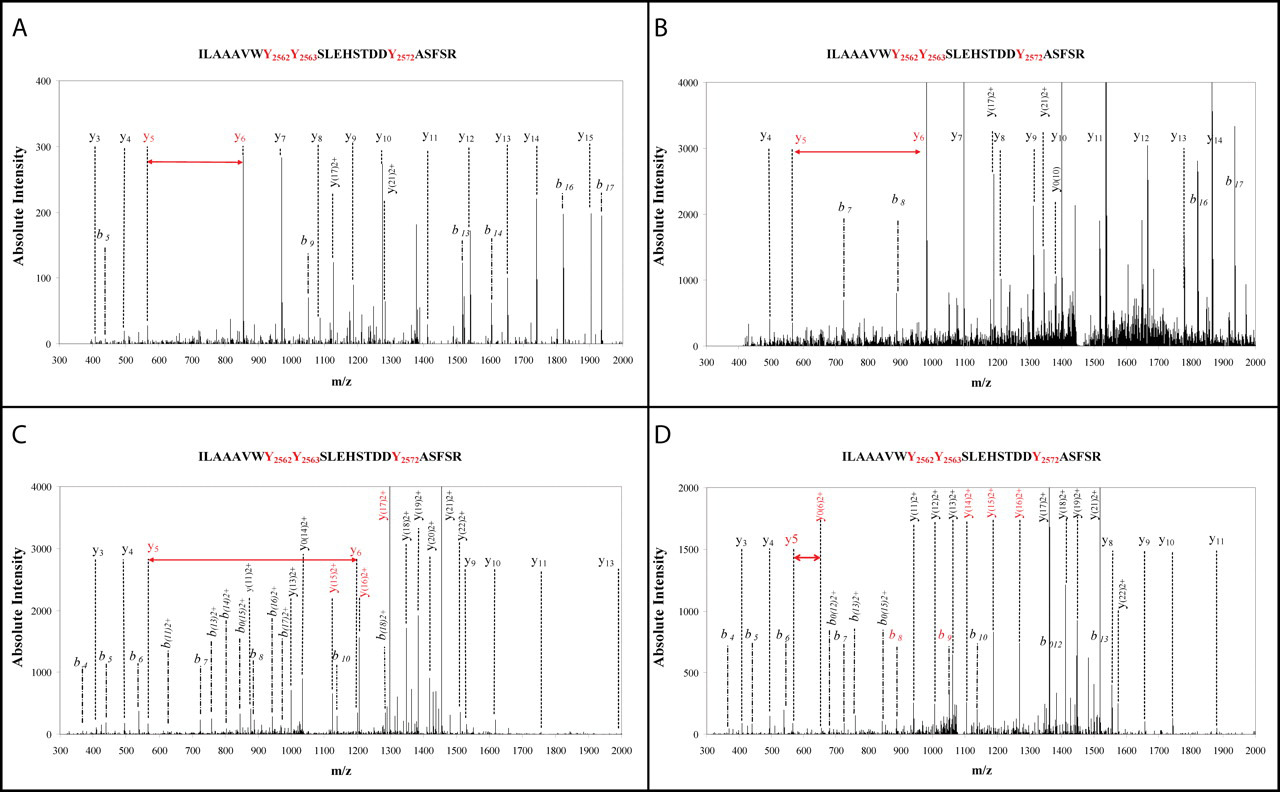
Dedieu, A. et al. Journal of Biological Chemistry, 2011.
Figure 2. Unambiguous Assignment of Four Different Iodination Patterns on Tyr2572.
FAQ
Q1: Can Low-Abundance Iodinated Proteins Be Detected?
A1: Yes. We utilize specific enrichment strategies, such as anti-iodinated peptide antibodies or iodine-labeling reactions, in combination with high-sensitivity mass spectrometry platforms to achieve accurate identification of low-abundance modifications.
Q2: What Information Is Included in the Data Deliverables?
A2: The deliverables include iodination modification sites, peptide sequences, spectral data, quantitative results, and functional annotations. All data are formatted to support downstream bioinformatics analysis and scientific publication requirements.
How to order?







