Hypromellose Acetate Succinate Analysis Service | Pharmaceutical Excipient
- Multidimensional Analytical Platforms: Comprehensive characterization of the physicochemical properties and functional attributes of HPMCAS by integrating chromatography, mass spectrometry, thermal analysis, spectroscopy, and rheology.
- High-Data-Quality: Deep data coverage with strict data quality control. An AI-powered bioinformatics platform integrates all Hypromellose Acetate Succinate Analysis Service data, providing clients with a comprehensive data report.
- Customized Analytical Solutions: Flexible design of tailored analytical strategies based on the specific application scenarios and project requirements of HPMCAS.
- Extended Applications: Analytical results are applicable not only to pharmaceutical formulations but also to cosmetics, food, and materials science.
- Solid Dispersion Preparation: HPMCAS can effectively improve the solubility and oral bioavailability of poorly soluble drugs, and its thermal and solubility analysis can provide a basis for process optimization.
- Controlled-Release and Sustained-Release Formulations: Rheological and thermal property studies determine their film-forming ability and release characteristics in controlled and sustained-release formulations.
- Drug Coating: Thermal stability, solubility, and compatibility testing of HPMCAS support the development of stable coating systems, improving drug appearance and release properties.
Hypromellose Acetate Succinate Analysis Service is a comprehensive analytical service for HPMCAS that covers chemical composition identification, thermal and physical property characterization, solubility and rheological studies, as well as compatibility evaluation with drugs, aiming to provide reliable data support for drug development, quality control, and industrial applications.
Hypromellose acetate succinate (HPMCAS) is a commonly used pharmaceutical excipient with good biocompatibility and solubility, and it is widely applied in solid dispersions, controlled-release formulations, and drug coatings. As an excipient, the quality and performance of HPMCAS directly affect drug stability, dissolution, and absorption in vivo. Therefore, systematic analysis of the physicochemical properties and compatibility of HPMCAS is of great importance for ensuring formulation quality consistency and optimizing manufacturing processes.
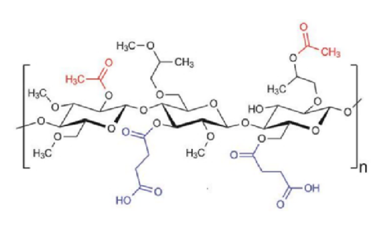
Yu D. et al. AAPS PharmSciTech. 2022.
Figure 1. Chemical structure of hypromellose acetate succinate (HPMCAS)
Services at MtoZ Biolabs
MtoZ Biolabs provides a systematic Hypromellose Acetate Succinate Analysis Service that covers its chemical, physical, and functional properties to help clients comprehensively evaluate the performance and applicability of this excipient in drug development.
1. Chemical Composition Analysis
Identification of the composition, impurities, and additives of HPMCAS using liquid chromatography mass spectrometry (LC-MS) and gas chromatography mass spectrometry (GC-MS).
2. Thermal Property Analysis
Evaluation of thermal stability, decomposition temperature, and glass transition temperature of HPMCAS by thermogravimetric analysis (TGA) and differential scanning calorimetry (DSC).
3. Solubility Testing
Determination of HPMCAS solubility in different solvent systems through solubility experiments.
4. Rheological Property Study
Assessment of viscosity, rheological curves, and rheological index of HPMCAS using rheological analysis.
5. Drug Compatibility Evaluation
Investigation of interactions between HPMCAS and different drug components using mass spectrometry and thermal analysis to identify potential chemical reactions and compatibility risks.
Service Advantages
Sample Submission Suggestions
1. Sample Types
Various sample types are accepted, including HPMCAS raw powder, solutions, or formulations containing HPMCAS.
2. Storage Conditions
Store at room temperature, protected from light, and avoid humid environments. For solution samples, low-temperature storage is recommended, and repeated freeze-thaw cycles should be avoided.
3. Additional Information
Please provide the HPMCAS grade, source, intended application, and formulation background to optimize the analytical strategy.
It is recommended to contact the MtoZ Biolabs technical team prior to sample submission to obtain detailed and tailored guidelines for sample preparation and submission.
Applications
Examples of applications of Hypromellose Acetate Succinate Analysis Service in the pharmaceutical Field:
How to order?







