Hydroxypropyl Cellulose Analysis Service | Pharmaceutical Excipient
Hydroxypropyl cellulose (HPC) is a nonionic cellulose ether obtained by partial substitution of cellulose molecules with hydroxypropyl groups. Its unique physicochemical properties, including water solubility, thermal reversibility, good film-forming ability, and surface activity, make it widely used in the pharmaceutical industry. HPC can serve not only as a binder and disintegrant for tablets but also as a film-coating material and controlled-release carrier, enhancing formulation stability, regulating drug release rate, and improving patient compliance.
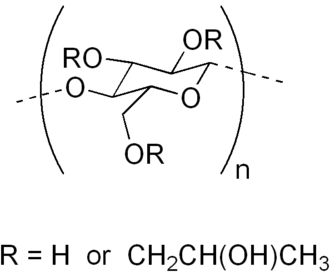
Figure 1. The Structure of Hydroxypropyl Cellulose
With increasingly stringent pharmacopeial standards and the continuous development of novel dosage forms, parameters such as the degree of substitution, molecular weight, viscosity, impurity levels, and application suitability of HPC have become critical quality attributes for pharmaceutical companies. Leveraging advanced analytical platforms and a professional team, MtoZ Biolabs offers a hydroxypropyl cellulose analysis service for research institutions and pharmaceutical companies to ensure the stability and reliability of excipients in compliance with both research and production requirements.
Services at MtoZ Biolabs
The quality evaluation of HPC typically involves multiple dimensions: structural identification, hydroxypropoxy substitution level, physicochemical properties, impurity control, and functional performance. MtoZ Biolabs has established a complete testing system based on international pharmacopeial requirements and advanced analytical methods. Using infrared spectroscopy, gas chromatography, nuclear magnetic resonance, thermal analysis, rheology, and elemental analysis, we can systematically assess the key quality attributes of HPC.
In response to clients’ research needs, we also offer performance studies under special conditions, such as solubility and phase separation behavior at different temperatures, or comparative analysis of samples with varying molecular weights. If you are interested in our service, please feel free to contact us.
Analysis Workflow
1. Sample Reception and Pretreatment: Verify sample information, label, and store appropriately.
2. Basic Analysis: Perform preliminary tests such as appearance, solubility, and moisture content.
3. Composition and Impurity Analysis: Conduct IR, GC, LOD, ROI, and heavy metal testing in accordance with pharmacopeial standards and client requirements.
4. Functional Performance Evaluation: Assess film-forming ability, swelling capacity, and disintegration performance according to application needs.
5. Data Review and Reporting: All results are quality-checked and compiled into a complete report.
Service Advantages
1. Comprehensive Testing Coverage
From structural identification and hydroxypropoxy content to physicochemical properties and impurity control, every critical attribute of HPC is systematically evaluated.
2. Compliance with Pharmacopeia and Research Needs
Our hydroxypropyl cellulose analysis service complies with USP, EP, JP, and other international pharmacopeias while also providing customized testing solutions tailored to specific client applications.
3. Multi-Platform Analytical Methods
With spectroscopy, chromatography, thermal analysis, and rheology techniques, we ensure the scientific accuracy and reliability of data.
4. Rigorous Quality Control System
All analytical processes are performed under standardized laboratory management, ensuring data accuracy, reproducibility, and traceability.
5. Professional Technical Support
Our team has extensive experience in excipient analysis and formulation development, helping clients interpret results and optimize R&D strategies.
Applications
1️⃣ Excipient screening and evaluation in drug development
2️⃣ Film-coating and disintegration studies in solid dosage forms
3️⃣ Quality data support for drug registration and regulatory submission
4️⃣ Consistency control during pharmaceutical manufacturing
5️⃣ Excipient import/export testing and compliance verification
FAQs
Q1: What is the typical turnaround time of hydroxypropyl cellulose analysis service?
Usually 2–3 weeks after sample receipt, depending on the number and complexity of tests.
Q2: Can the test results be used for regulatory submission?
Yes, the report complies with pharmacopeial and regulatory requirements and can be used as part of registration and quality documentation.
Sample Submission Suggestions
1. Sample Type: Powder or granules.
2. Sample Quantity: Recommended 10–20 g.
3. Packaging: Sealed and moisture-proof.
4. Transportation: Ambient conditions unless otherwise specified.
5. Information: Include sample source, batch number, and intended application.
Deliverables
1. Comprehensive test report (methods, raw data, spectra/chromatograms, and calculations)
2. Physicochemical property and impurity analysis results
3. Functional performance data and suitability evaluation
4. Interpretation of results and reference recommendations
5. Raw chromatographic/spectral files
As an indispensable excipient in pharmaceutical formulations, the quality and stability of hydroxypropyl cellulose directly impact drug safety and efficacy. With advanced analytical platforms and a professional team, MtoZ Biolabs provides systematic and comprehensive hydroxypropyl cellulose analysis service to support drug development and quality control. Contact us today to obtain high-quality excipient analysis solutions and work with us to advance efficient pharmaceutical development.
How to order?







